Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate
- PMID: 11159992
- PMCID: PMC97976
- DOI: 10.1128/IAI.69.2.968-976.2001
Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate
Abstract
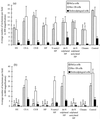
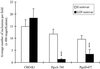
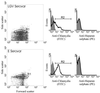
The ability of heparan sulfate, heparin, and other glycosaminoglycans to inhibit the infectivity of Chlamydia trachomatis serovars E and LGV was examined using a simple competitive inhibition assay with three cell types from the human female reproductive tract, including primary human endosalpingeal cells. With the majority of the glycosaminoglycans tested, LGV was more significantly inhibited than serovar E. We have compared chlamydial infectivity between a wild-type Chinese hamster ovary cell line and two glycosaminoglycan-deficient cell lines. LGV was shown to be unable to infect heparan sulfate-deficient and GAG-deficient Chinese hamster ovary cell lines, whereas the E serovar infected these cells as efficiently as the control (nondeficient) cells. These two sets of experiments confirmed that serovar LGV is more dependent on a heparan sulfate-related mechanism of infectivity than is serovar E. This is further supported by the fact that attempts to purify a heparan sulfate-like molecule from either serovar cultured in glycosaminoglycan-deficient cell lines were nonproductive. Previous reports have suggested that chlamydia are able to produce a heparan sulfate-like molecule that is important for attachment and infectivity. We have attempted to detect possible binding of a specific heparan sulfate antibody to C. trachomatis by flow cytometry. Results showed no binding of the heparan sulfate antibody to C. trachomatis serovar LGV or E. Our results strongly indicate that chlamydiae do not produce a heparan sulfate-like molecule but rather use host cell heparan sulfate in order to infect cells.
Figures


References
-
- Bernfield M, Gotte M, Park P P, Reizes O, Fitzgerald M L, Lincenum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Brieger D, Dawes J. Production method affects the pharmacokinetic and ex vivo biological properties of low molecular weight heparins. Thromb Haemost. 1997;77:317–322. - PubMed
-
- Cates W, Wasserheit J N. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991;164:1771–1781. - PubMed
-
- Cesar B, Gutierrez-Martin C B, Ojcius D M, Hsia R, Hellio R, Bavoil P M, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci to HeLa cells. Microb Pathog. 1997;22:47–57. - PubMed
-
- Chen J C R, Stephens R S. Trachoma and LGV biovars of Chlamydia trachomatis share the same glycosaminoglycan-dependent mechanism for infection of eukaryotic cells. Mol Microbiol. 1994;11:501–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

