Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax
- PMID: 11160005
- PMCID: PMC97989
- DOI: 10.1128/IAI.69.2.1084-1092.2001
Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax
Abstract
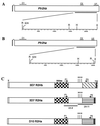
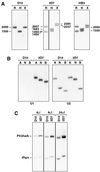
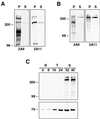
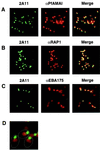
Plasmodium falciparum infections can be fatal, while P. vivax infections usually are not. A possible factor involved in the greater virulence of P. falciparum is that this parasite grows in red blood cells (RBCs) of all maturities whereas P. vivax is restricted to growth in reticulocytes, which represent only approximately 1% of total RBCs in the periphery. Two proteins, expressed at the apical end of the invasive merozoite stage from P. vivax, have been implicated in the targeting of reticulocytes for invasion by this parasite. A search of the P. falciparum genome databases has identified genes that are homologous to the P. vivax rbp-1 and -2 genes. Two of these genes are virtually identical over a large region of the 5' end but are highly divergent at the 3' end. They encode high-molecular-mass proteins of >300 kDa that are expressed in late schizonts and localized to the apical end of the merozoite. To test a potential role in merozoite invasion of RBCs, we analyzed the ability of these proteins to bind to mature RBCs and reticulocytes. No binding to mature RBCs or cell preparations enriched for reticulocytes was detected. We identified a parasite clone that lacks the gene for one of these proteins, showing that the gene is not required for normal in vitro growth. Antibodies to these proteins can inhibit merozoite invasion of RBCs.
Figures



References
-
- Borre M B, Owen C A, Keen J K, Sinha K A, Holder A A. Multiple genes code for high-molecular-mass rhoptry proteins of Plasmodium yoelii. Mol Biochem Parasitol. 1995;70:149–155. - PubMed
-
- Dolan S A, Proctor J L, Alling D W, Okubo Y, Wellems T E, Miller L H. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. - PubMed
-
- Freeman R R, Trejdosiewicz A J, Cross G A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980;284:366–368. - PubMed
-
- Galinski M R, Medina C C, Ingravallo P, Barnwell J W. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69:1213–1226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

