Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-beta2 microglobulin-null mice
- PMID: 11160136
- PMCID: PMC199177
- DOI: 10.1172/JCI11519
Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-beta2 microglobulin-null mice
Abstract
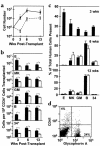
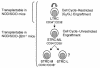
Increasing use of purified or cultured human hematopoietic cells as transplants has revealed an urgent need for better methods to predict the speed and durability of their engraftment potential. We now show that NOD/SCID-beta2 microglobulin-null (NOD/SCID-beta2m-/-) mice are sequentially engrafted by two distinct and previously unrecognized populations of transplantable human short-term repopulating hematopoietic cells (STRCs), neither of which efficiently engraft NOD/SCID mice. One is predominantly CD34+CD38+ and is myeloid-restricted; the other is predominantly CD34+CD38- and has broader lymphomyeloid differentiation potential. In contrast, the long-term repopulating human cells that generate lymphoid and myeloid progeny in NOD/SCID mice engraft and self-renew in NOD/SCID-beta2m-/- mice equally efficiently. In short-term expansion cultures of adult bone marrow cells, myeloid-restricted STRCs were preferentially amplified (greater than tenfold) and, interestingly, both types of STRC were found to be selectively elevated in mobilized peripheral blood harvests. These results suggest an enhanced sensitivity of STRCs to natural killer cell-mediated rejection. They also provide new in vivo assays for different types of human STRC that may help to predict the engraftment potential of clinical transplants and facilitate future investigation of early stages of human hematopoietic stem cell differentiation.
Figures


References
-
- Gluckman E, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. - PubMed
-
- Pflumio F, et al. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. - PubMed
-
- Wang JCY, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. - PubMed
-
- van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055–3061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

