LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction
- PMID: 11160139
- PMCID: PMC199173
- DOI: 10.1172/JCI10832
LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction
Abstract
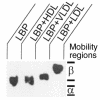
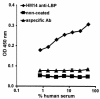
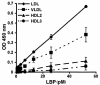
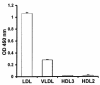
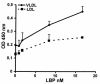
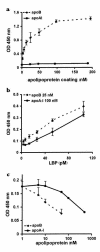
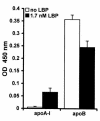
LPS-binding protein (LBP) and serum lipoproteins cooperate in reducing the toxic properties of LPS. In the present study, we demonstrate that LBP circulates in association with LDL and VLDL in healthy persons. ApoB was found to account at least in part for the interaction of LBP with LDL and VLDL. Although LBP interacted with purified apoA-I in vitro, no association of LBP with apoA-I or HDL was found in serum. Consistent with the observed association of LBP with LDL and VLDL, these lipoproteins also were demonstrated to be the predominant LPS-binding lipoproteins. Most interestingly, the association of LBP with LDL and VLDL strongly enhanced the capacity of these lipoproteins to bind LPS. Because this function of LBP is of utmost importance during infection, the association of LBP and LPS with lipoproteins was also studied in serum from septic patients. In septic serum containing high LBP levels and a markedly altered lipoprotein spectrum, most of the LBP is associated with LDL and VLDL, although some LBP appeared to circulate free from lipoproteins. Also in this serum, LPS was found to bind predominantly to LDL and VLDL. The observed binding of LBP and LPS to LDL and VLDL, as well as the LBP-dependent incorporation of LPS into these lipoproteins, emphasizes a crucial role for circulating LBP-LDL/VLDL complexes in the scavenging of LPS.
Figures
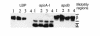

References
-
- Read TE, et al. The protective effect of serum lipoproteins against bacterial lipopolysaccharide. Eur Heart J. 1993;14:125–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

