Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4
- PMID: 11160142
- PMCID: PMC199172
- DOI: 10.1172/JCI10559
Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4
Abstract
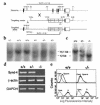
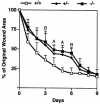
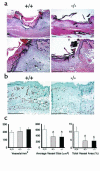
The syndecans make up a family of transmembrane heparan sulfate proteoglycans that act as coreceptors with integrins and growth factor tyrosine kinase receptors. Syndecan-4 is upregulated in skin dermis after wounding, and, in cultured fibroblasts adherent to the ECM protein fibronectin, this proteoglycan signals cooperatively with beta1 integrins. In this study, we generated mice in which the syndecan-4 gene was disrupted by homologous recombination in embryonic stem cells to test the hypothesis that syndecan-4 contributes to wound repair. Mice heterozygous or homozygous for the disrupted syndecan-4 gene are viable, fertile, and macroscopically indistinguishable from wild-type littermates. Compared with wild-type littermates, mice heterozygous or homozygous for the disrupted gene have statistically significant delayed healing of skin wounds and impaired angiogenesis in the granulation tissue. These results indicate that syndecan-4 is an important cell-surface receptor in wound healing and angiogenesis and that syndecan-4 is haplo-insufficient in these processes.
Figures

References
-
- Clark RA. Biology of dermal wound repair. Dermatol Clin. 1993;11:647–666. - PubMed
-
- Kyriakides TR, Tam JWY, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. - PubMed
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

