Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance
- PMID: 11160162
- PMCID: PMC199192
- DOI: 10.1172/JCI10660
Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance
Abstract
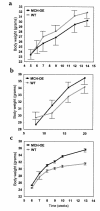
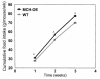
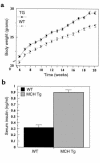
Several lines of investigation suggest that the hypothalamic neuropeptide melanin-concentrating hormone (MCH) regulates body weight in mammals. Obese mice lacking functional leptin overexpress the MCH message in the fed or fasted state. Acute intracerebroventricular injection of MCH increases energy intake in rats. Mice lacking the MCH gene are lean. To test the hypothesis that chronic overexpression of MCH in mice causes obesity, we produced transgenic mice that overexpress MCH (MCH-OE) in the lateral hypothalamus at approximately twofold higher levels than normal mice. On the FVB genetic background, homozygous transgenic animals fed a high-fat diet ate 10% more and were 12% heavier at 13 weeks of age than wild-type animals, and they had higher systemic leptin levels. Blood glucose levels were higher both preprandially and after an intraperitoneal glucose injection. MCH-OE animals were insulin-resistant, as demonstrated by markedly higher plasma insulin levels and a blunted response to insulin; MCH-OE animals had only a 5% decrease in blood glucose after insulin administration, compared with a 31% decrease in wild-type animals. MCH-OE animals also exhibited a twofold increase in islet size. To evaluate the contribution of genetic background to the predisposition to obesity seen in MCH-OE mice, the transgene was bred onto the C57BL/6J background. Heterozygote C57BL/6J mice expressing the transgene showed increased body weight on a standard diet, confirming that MCH overexpression can lead to obesity.
Figures





References
-
- Flier JS, Maratos-Flier E. Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998; 92:437–440. - PubMed
-
- Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropeptides. 1999; 33:339–349. - PubMed
-
- Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996; 380:243–247. - PubMed
-
- Ludwig DS, et al. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998; 274:E627–E633. - PubMed
-
- Bittencourt JC, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992; 319:218–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

