G-protein inhibition of N- and P/Q-type calcium channels: distinctive elementary mechanisms and their functional impact
- PMID: 11160384
- PMCID: PMC6762231
- DOI: 10.1523/JNEUROSCI.21-04-01137.2001
G-protein inhibition of N- and P/Q-type calcium channels: distinctive elementary mechanisms and their functional impact
Erratum in
- J Neurosci 2001 Mar 15;21(6):1a
Abstract
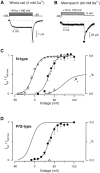
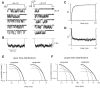
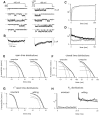
Voltage-dependent G-protein inhibition of presynaptic Ca(2+) channels is a key mechanism for regulating synaptic efficacy. G-protein betagamma subunits produce such inhibition by binding to and shifting channel opening patterns from high to low open probability regimes, known respectively as "willing" and "reluctant" modes of gating. Recent macroscopic electrophysiological data hint that only N-type, but not P/Q-type channels can open in the reluctant mode, a distinction that could enrich the dimensions of synaptic modulation arising from channel inhibition. Here, using high-resolution single-channel recording of recombinant channels, we directly distinguished this core contrast in the prevalence of reluctant openings. Single, inhibited N-type channels manifested relatively infrequent openings of submillisecond duration (reluctant openings), which differed sharply from the high-frequency, millisecond gating events characteristic of uninhibited channels. By contrast, inhibited P/Q-type channels were electrically silent at the single-channel level. The functional impact of the differing inhibitory mechanisms was revealed in macroscopic Ca(2+) currents evoked with neuronal action potential waveforms (APWs). Fitting with a change in the manner of opening, inhibition of such N-type currents produced both decreased current amplitude and temporally advanced waveform, effects that would not only reduce synaptic efficacy, but also influence the timing of synaptic transmission. On the other hand, inhibition of P/Q-type currents evoked by APWs showed diminished amplitude without shape alteration, as expected from a simple reduction in the number of functional channels. Variable expression of N- and P/Q-type channels at spatially distinct synapses therefore offers the potential for custom regulation of both synaptic efficacy and synchrony, by G-protein inhibition.
Figures



Similar articles
-
Differential occurrence of reluctant openings in G-protein-inhibited N- and P/Q-type calcium channels.J Gen Physiol. 2000 Feb;115(2):175-92. doi: 10.1085/jgp.115.2.175. J Gen Physiol. 2000. PMID: 10653895 Free PMC article.
-
G protein modulation of recombinant P/Q-type calcium channels by regulators of G protein signalling proteins.J Physiol. 2000 Oct 1;528 Pt 1(Pt 1):65-77. doi: 10.1111/j.1469-7793.2000.00065.x. J Physiol. 2000. PMID: 11018106 Free PMC article.
-
Decoding of synaptic voltage waveforms by specific classes of recombinant high-threshold Ca(2+) channels.J Physiol. 2003 Dec 1;553(Pt 2):473-88. doi: 10.1113/jphysiol.2003.051110. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500770 Free PMC article.
-
Neurotransmitter modulation of neuronal calcium channels.J Bioenerg Biomembr. 2003 Dec;35(6):477-89. doi: 10.1023/b:jobb.0000008021.55853.18. J Bioenerg Biomembr. 2003. PMID: 15000517 Review.
-
Regulation of Ca(V)2 calcium channels by G protein coupled receptors.Biochim Biophys Acta. 2013 Jul;1828(7):1629-43. doi: 10.1016/j.bbamem.2012.10.004. Epub 2012 Oct 12. Biochim Biophys Acta. 2013. PMID: 23063655 Free PMC article. Review.
Cited by
-
Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons.J Neurosci. 2007 Dec 5;27(49):13420-9. doi: 10.1523/JNEUROSCI.1709-07.2007. J Neurosci. 2007. PMID: 18057200 Free PMC article.
-
Light control of G protein signaling pathways by a novel photopigment.PLoS One. 2018 Oct 1;13(10):e0205015. doi: 10.1371/journal.pone.0205015. eCollection 2018. PLoS One. 2018. PMID: 30273391 Free PMC article.
-
Presynaptic Ca2+ influx and vesicle exocytosis at the mouse endbulb of Held: a comparison of two auditory nerve terminals.J Physiol. 2011 Sep 1;589(17):4301-20. doi: 10.1113/jphysiol.2011.209189. Epub 2011 Jul 11. J Physiol. 2011. PMID: 21746778 Free PMC article.
-
Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties.Heliyon. 2024 Feb 21;10(5):e26656. doi: 10.1016/j.heliyon.2024.e26656. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434323 Free PMC article.
-
Design and Applications of Genetically-Encoded Voltage-Dependent Calcium Channel Inhibitors.Handb Exp Pharmacol. 2023;279:139-155. doi: 10.1007/164_2023_656. Handb Exp Pharmacol. 2023. PMID: 37306815 Review.
References
-
- Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983;306:436–441. - PubMed
-
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann NY Acad Sci. 1991;635:365–381. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- Bertram R, Sherman A, Stanley EF. Single-domain/bound calcium hypothesis of transmitter release and facilitation. J Neurophysiol. 1996;75:1919–1931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
