The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling
- PMID: 11160390
- PMCID: PMC6762247
- DOI: 10.1523/JNEUROSCI.21-04-01203.2001
The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling
Abstract
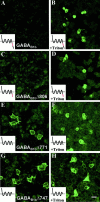
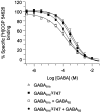
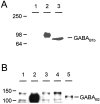
GABA(B) receptors are G-protein-coupled receptors that mediate slow synaptic inhibition in the brain and spinal cord. These receptors are heterodimers assembled from GABA(B1) and GABA(B2) subunits, neither of which is capable of producing functional GABA(B) receptors on homomeric expression. GABA(B1,) although able to bind GABA, is retained within the endoplasmic reticulum (ER) when expressed alone. In contrast, GABA(B2) is able to access the cell surface when expressed alone but does not couple efficiently to the appropriate effector systems or produce any detectable GABA-binding sites. In the present study, we have constructed chimeric and truncated GABA(B1) and GABA(B2) subunits to explore further GABA(B) receptor signaling and assembly. Removal of the entire C-terminal intracellular domain of GABA(B1) results in plasma membrane expression without the production of a functional GABA(B) receptor. However, coexpression of this truncated GABA(B1) subunit with either GABA(B2) or a truncated GABA(B2) subunit in which the C terminal has also been removed is capable of functional signaling via G-proteins. In contrast, transferring the entire C-terminal tail of GABA(B1) to GABA(B2) leads to the ER retention of the GABA(B2) subunit when expressed alone. These results indicate that the C terminal of GABA(B1) mediates the ER retention of this protein and that neither of the C-terminal tails of GABA(B1) or GABA(B2) is an absolute requirement for functional coupling of heteromeric receptors. Furthermore although GABA(B1) is capable of producing GABA-binding sites, GABA(B2) is of central importance in the functional coupling of heteromeric GABA(B) receptors to G-proteins and the subsequent activation of effector systems.
Figures



References
-
- Benke D, Honer M, Michel C, Bettler B, Mohler H. Gamma-aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem. 1999;274:27323–27330. - PubMed
-
- Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. - PubMed
-
- Bowen WP, Jerman JC. Nonlinear regression using spreadsheets. Trends Pharmacol Sci. 1995;16:413–417. - PubMed
-
- Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. - PubMed
-
- Bowery NG, Enna SJ. Gamma-aminobutyric acid (B) receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
