Stimulation of nitric oxide-cGMP pathway excites striatal cholinergic interneurons via protein kinase G activation
- PMID: 11160411
- PMCID: PMC6762226
- DOI: 10.1523/JNEUROSCI.21-04-01393.2001
Stimulation of nitric oxide-cGMP pathway excites striatal cholinergic interneurons via protein kinase G activation
Abstract
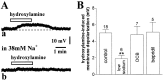
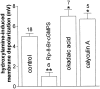
Conflicting data have been collected so far on the action of nitric oxide (NO) on cholinergic interneurons of the striatum. In the present in vitro electrophysiological study, we reported that intracellularly recorded striatal cholinergic interneurons are excited by both hydroxylamine and S-nitroso-N-acetylpenicillamine, two NO donors. This excitation persisted unchanged in the presence of glutamate, dopamine, and substance P receptor antagonists as well as after blockade of tetrodotoxin (TTX)- and calcium channel-sensitive transmitter release, suggesting that NO produces its effects by modulating directly resting ion conductances in the somatodendritic region of striatal cholinergic cells. The depolarizing effect of hydroxylamine was greatly reduced by lowering external concentrations of sodium ions (from 126 to 38 mm) and did not reverse polarity in the voltage range from -120 to -40 mV. The sodium transporter blockers bepridil and 3',4'-dichlorobenzamil were conversely ineffective in preventing NO-induced membrane depolarization. Intracellular cGMP elevation is required for the action of hydroxylamine on striatal cholinergic cells, as demonstrated by the findings that the membrane depolarization produced by this pharmacological agent was prevented by bath and intracellular application of two inhibitors of soluble guanylyl cyclase and was mimicked and occluded by zaprinast, a cGMP phosphodiesterase inhibitor. Finally, intracellular Rp-8-Br-cGMPS, a protein kinase G (PKG) inhibitor, blocked the hydroxylamine-induced membrane depolarization of cholinergic interneurons, whereas both okadaic acid and calyculin A, two protein phosphatase inhibitors, enhanced it, indicating that intracellular PKG and phosphatases oppositely regulate the sensitivity of striatal cholinergic interneurons to NO. The characterization of the cellular mechanisms involved in the regulation of striatal interneuron activity is a key step for the understanding of the role of these cells in striatal microcircuitry.
Figures




References
-
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. - PubMed
-
- Black MD, Matthews EK, Humphrey PP. The effects of a photosensitive nitric oxide donor on basal and electrically-stimulated dopamine efflux from the rat striatum in vitro. Neuropharmacology. 1994;33:1357–1365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
