Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons
- PMID: 11160424
- PMCID: PMC6763829
- DOI: 10.1523/JNEUROSCI.21-02-00434.2001
Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons
Abstract
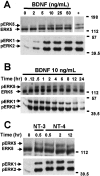
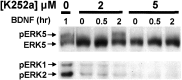
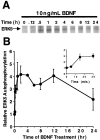
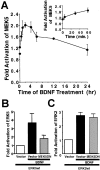
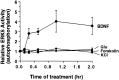
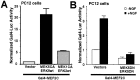
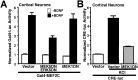
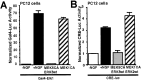
Activation of the extracellular signal-regulated kinase 1 (ERK1) and ERK2 by neurotrophins, neuronal activity, or cAMP has been strongly implicated in differentiation, survival, and adaptive responses of neurons during development and in the adult brain. Recently, a new member of the mitogen-activated protein (MAP) kinase family, ERK5, was discovered. Like ERK1 and ERK2, ERK5 is expressed in neurons, and ERK5 stimulation by epidermal growth factor is blocked by the MAP kinase/ERK kinase 1 (MEK1) inhibitors PD98059 and U0126. This suggests the interesting possibility that some of the functions attributed to ERK1/2 may be mediated by ERK5. However, the regulatory properties of ERK5 in primary cultured neurons have not been reported. Here we examined the regulation of ERK5 signaling in primary cultured cortical neurons. Our data demonstrate that, similar to ERK1/2, ERK5 is activated by neurotrophins including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4. BDNF stimulation of ERK5 required the activity of MEK5. Surprisingly, ERK5 was not stimulated by cAMP or neuronal activity induced by glutamate or membrane depolarization. In contrast to ERK1/2, ERK5 strongly activated the transcriptional activity of myocyte enhancer factor 2C (MEF2C) in pheochromocytoma 12 (PC12) cells and was required for neurotrophin stimulation of MEF2C transcription in both PC12 cells and cortical neurons. Furthermore, ERK1/2, but not ERK5, induced transcription from Elk1 and the cAMP/ Ca(2+) response element in PC12 cells. Our data suggest that mechanisms for regulation of ERK5 and downstream transcriptional pathways regulated by ERK5 are distinct from those of ERK1/2 in neurons. Furthermore, ERK5 is the first MAP kinase identified whose activity is stimulated by neurotrophins but not by neuronal activity.
Figures

References
-
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. - PubMed
-
- Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1 (BMK1). J Biol Chem. 1997;272:20389–20394. - PubMed
-
- Ahn NG, Seger R, Bratlien RL, Krebs EG. Growth factor-stimulated phosphorylation cascades: activation of growth factor-stimulated MAP kinase. Ciba Found Symp 164 1992. 113 126; discussion 126–131. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
