Murine cytomegalovirus open reading frame M27 plays an important role in growth and virulence in mice
- PMID: 11160668
- PMCID: PMC114079
- DOI: 10.1128/JVI.75.4.1697-1707.2001
Murine cytomegalovirus open reading frame M27 plays an important role in growth and virulence in mice
Abstract
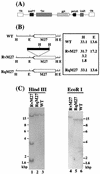
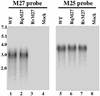
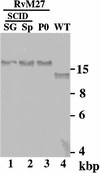
Using a Tn3-based transposon mutagenesis approach, we have generated a pool of murine cytomegalovirus (MCMV) mutants. In this study, one of the mutants, RvM27, which contained the transposon sequence at open reading frame M27, was characterized both in tissue culture and in immunocompetent BALB/c mice and immunodeficient SCID mice. Our results suggest that the M27 carboxyl-terminal sequence is dispensable for viral replication in vitro. Compared to the wild-type strain and a rescued virus that restored the M27 region, RvM27 was attenuated in growth in both BALB/c and SCID mice that were intraperitoneally infected with the viruses. Specifically, the titers of RvM27 in the salivary glands, lungs, spleens, livers, and kidneys of the infected SCID mice at 21 days postinfection were 50- to 500-fold lower than those of the wild-type virus and the rescued virus. Moreover, the virulence of the mutant virus appeared to be attenuated, because no deaths occurred among SCID mice infected with RvM27 for up to 37 days postinfection, while all the animals infected with the wild-type and rescued viruses died within 27 days postinfection. Our observations provide the first direct evidence to suggest that a disruption of M27 expression results in reduced viral growth and attenuated viral virulence in vivo in infected animals. Moreover, these results suggest that M27 is a viral determinant required for optimal MCMV growth and virulence in vivo and provide insight into the functions of the M27 homologues found in other animal and human CMVs as well as in other betaherpesviruses.
Figures

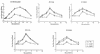

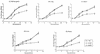
References
-
- Boname J M, Chantler J K. Characterization of a strain of murine cytomegalovirus which fails to grow in the salivary glands of mice. J Gen Virol. 1992;73:2021–2029. - PubMed
-
- Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2493–2523.
-
- Brune W, Menard C, Hobom U, Odenbreit S, Messerle M, Koszinowski U H. Rapid identification of essential and nonessential herpesvirus genes by direct transposon mutagenesis. Nat Biotechnol. 1999;17:360–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

