Specific interaction of hepatitis C virus protease/helicase NS3 with the 3'-terminal sequences of viral positive- and negative-strand RNA
- PMID: 11160669
- PMCID: PMC114080
- DOI: 10.1128/JVI.75.4.1708-1721.2001
Specific interaction of hepatitis C virus protease/helicase NS3 with the 3'-terminal sequences of viral positive- and negative-strand RNA
Abstract
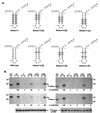
The hepatitis C virus (HCV)-encoded protease/helicase NS3 is likely to be involved in viral RNA replication. We have expressed and purified recombinant NS3 (protease and helicase domains) and Delta pNS3 (helicase domain only) and examined their abilities to interact with the 3'-terminal sequence of both positive and negative strands of HCV RNA. These regions of RNA were chosen because initiation of RNA synthesis is likely to occur at or near the 3' untranslated region (UTR). The results presented here demonstrate that NS3 (and Delta pNS3) interacts efficiently and specifically with the 3'-terminal sequences of both positive- and negative-strand RNA but not with the corresponding complementary 5'-terminal RNA sequences. The interaction of NS3 with the 3'-terminal negative strand [called 3'(-) UTR(127)] was specific in that only homologous (and not heterologous) RNA competed efficiently in the binding reaction. A predicted stem-loop structure present at the 3' terminus (nucleotides 5 to 20 from the 3' end) of the negative-strand RNA appears to be important for NS3 binding to the negative-strand UTR. Deletion of the stem-loop structure almost totally impaired NS3 (and Delta pNS3) binding. Additional mutagenesis showed that three G-C pairs within the stem were critical for helicase-RNA interaction. The data presented here also suggested that both a double-stranded structure and the 3'-proximal guanosine residues in the stem were important determinants of protein binding. In contrast to the relatively stringent requirement for 3'(-) UTR binding, specific interaction of NS3 (or Delta pNS3) with the 3'-terminal sequences of the positive-strand RNA [3'(+) UTR] appears to require the entire 3'(+) UTR of HCV. Deletion of either the 98-nucleotide 3'-terminal conserved region or the 5' half sequence containing the variable region and the poly(U) and/or poly(UC) stretch significantly impaired RNA-protein interaction. The implication of NS3 binding to the 3'-terminal sequences of viral positive- and negative-strand RNA in viral replication is discussed.
Figures



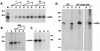
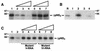

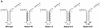
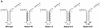
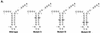
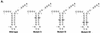
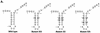

References
-
- Banerjee R, Igo M, Izumi R, Datta U, Dasgupta A. In vitro replication of RNA viruses. In: Cann A J, editor. RNA viruses. Oxford, United Kingdom: Oxford University Press; 2000. pp. 141–178.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

