Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity
- PMID: 11160682
- PMCID: PMC114093
- DOI: 10.1128/JVI.75.4.1834-1841.2001
Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity
Abstract
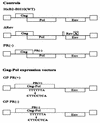
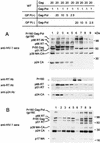
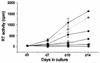
Production of the human immunodeficiency virus type 1 (HIV-1) Gag-Pol precursor protein results from a -1 ribosomal frameshifting event. In infected cells, this generates Gag and Gag-Pol in a ratio that is estimated to be 20:1, a ratio that is conserved among retroviruses. To examine the impact of this ratio on HIV-1 replication and viral assembly, we altered the Gag/Gag-Pol ratio in virus-producing cells by cotransfecting HIV-1 proviral DNA with an HIV-1 Gag-Pol expression vector. Two versions of the Gag-Pol expression vector were used; one contains an active protease [PR(+)], and the other contains an inactive protease [PR(-)]. In an attempt to produce viral particles with Gag/Gag-Pol ratios ranging from 20:21 to 20:1 (wild type), 293T cells were cotransfected with various ratios of wild-type proviral DNA and proviral DNA from either Gag-Pol expression vector. Viral particles derived from cells with altered Gag/Gag-Pol ratios via overexpression of PR(-) Gag-Pol showed a ratio-dependent defect in their virion protein profiles. However, the defects in virion infectivity were independent of the nature of the Gag-Pol expression vector, i.e., PR(+) or PR(-). Based on equivalent input of reverse transcriptase activity, we estimated that HIV-1 infectivity was reduced 250- to 1,000-fold when the Gag/Gag-Pol ratio in the virion-producing cells was altered from 20:1 to 20:21. Although virion RNA packaging was not affected by altering Gag/Gag-Pol ratios, changing the ratio from 20:1 to 20:21 progressively reduced virion RNA dimer stability. The impact of the Gag/Gag-Pol ratio on virion RNA dimerization was amplified when the Gag-Pol PR(-) expression vector was expressed in virion-producing cells. Virions produced from cells expressing Gag and Gag-Pol PR(-) in a 20:21 ratio contained mainly monomeric RNA. Our observations provide the first direct evidence that, in addition to proteolytic processing, the ratio of Gag/Gag-Pol proteins is also important for RNA dimerization and that stable RNA dimers are not required for encapsidation of genomic RNA in HIV-1.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

