Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress
- PMID: 11160822
- PMCID: PMC30567
- DOI: 10.1091/mbc.12.1.53
Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress
Abstract
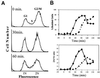
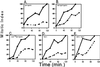
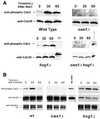
Exposure of yeast cells to an increase in external osmolarity induces a temporary growth arrest. Recovery from this stress is mediated by the accumulation of intracellular glycerol and the transcription of several stress response genes. Increased external osmolarity causes a transient accumulation of 1N and 2N cells and a concomitant depletion of S phase cells. Hypertonic stress triggers a cell cycle delay in G2 phase cells that appears distinct from the morphogenesis checkpoint, which operates in early S phase cells. Hypertonic stress causes a decrease in CLB2 mRNA, phosphorylation of Cdc28p, and inhibition of Clb2p-Cdc28p kinase activity, whereas Clb2 protein levels are unaffected. Like the morphogenesis checkpoint, the osmotic stress-induced G2 delay is dependent upon the kinase Swe1p, but is not tightly correlated with inhibition of Clb2p-Cdc28p kinase activity. Thus, deletion of SWE1 does not prevent the hypertonic stress-induced inhibition of Clb2p-Cdc28p kinase activity. Mutation of the Swe1p phosphorylation site on Cdc28p (Y19) does not fully eliminate the Swe1p-dependent cell cycle delay, suggesting that Swe1p may have functions independent of Cdc28p phosphorylation. Conversely, deletion of the mitogen-activated protein kinase HOG1 does prevent Clb2p-Cdc28p inhibition by hypertonic stress, but does not block Cdc28p phosphorylation or alleviate the cell cycle delay. However, Hog1p does contribute to proper nuclear segregation after hypertonic stress in cells that lack Swe1p. These results suggest a hypertonic stress-induced cell cycle delay in G2 phase that is mediated in a novel way by Swe1p in cooperation with Hog1p.
Figures



References
-
- Albertyn J, Hohmann S, Prior BA. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr Genet. 1994a;25:12–18. - PubMed
-
- Albertyn J, Hohmann S, Thevelein JM, Prior BA. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994b;14:4135–4144. - PMC - PubMed
-
- Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

