An investigation of the storage and biosynthesis of phenylpropenes in sweet basil
- PMID: 11161012
- PMCID: PMC64856
- DOI: 10.1104/pp.125.2.539
An investigation of the storage and biosynthesis of phenylpropenes in sweet basil
Abstract
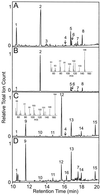
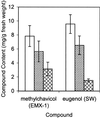
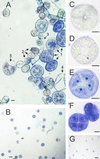
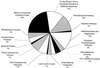
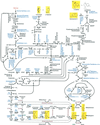
Plants that contain high concentrations of the defense compounds of the phenylpropene class (eugenol, chavicol, and their derivatives) have been recognized since antiquity as important spices for human consumption (e.g. cloves) and have high economic value. Our understanding of the biosynthetic pathway that produces these compounds in the plant, however, has remained incomplete. Several lines of basil (Ocimum basilicum) produce volatile oils that contain essentially only one or two specific phenylpropene compounds. Like other members of the Lamiaceae, basil leaves possess on their surface two types of glandular trichomes, termed peltate and capitate glands. We demonstrate here that the volatile oil constituents eugenol and methylchavicol accumulate, respectively, in the peltate glands of basil lines SW (which produces essentially only eugenol) and EMX-1 (which produces essentially only methylchavicol). Assays for putative enzymes in the biosynthetic pathway leading to these phenylpropenes localized many of the corresponding enzyme activities almost exclusively to the peltate glands in leaves actively producing volatile oil. An analysis of an expressed sequence tag database from leaf peltate glands revealed that known genes for the phenylpropanoid pathway are expressed at very high levels in these structures, accounting for 13% of the total expressed sequence tags. An additional 14% of cDNAs encoded enzymes for the biosynthesis of S-adenosyl-methionine, an important substrate in the synthesis of many phenylpropenes. Thus, the peltate glands of basil appear to be highly specialized structures for the synthesis and storage of phenylpropenes, and serve as an excellent model system to study phenylpropene biosynthesis.
Figures




References
-
- Adams S, Weidenborner M. Mycelial deformations of Cladosporium herbarum due to the application of eugenol or carvacrol. J Essential Oil Res. 1996;8:535–540.
-
- Anterola AM, van Rensburg H, van Heerden PS, Davin LB, Lewis NG. Multi-site modulation of flux during monolignol formation in loblolly pine (Pinus taeda) Biochem Biophys Res Commun. 1999;261:652–657. - PubMed
-
- Bala S, Sukul N. Systemic nematocidal effect of eugenol. Nematropica. 1987;17:219–222.
-
- Bara M, Vanetti M. Antimicrobial effect of spices on the growth of Yersinia enterocolitica. J Herb Spice Med Plant. 1995;3:51–58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

