The 5' end of the pea ferredoxin-1 mRNA mediates rapid and reversible light-directed changes in translation in tobacco
- PMID: 11161034
- PMCID: PMC64878
- DOI: 10.1104/pp.125.2.770
The 5' end of the pea ferredoxin-1 mRNA mediates rapid and reversible light-directed changes in translation in tobacco
Abstract
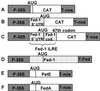
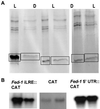
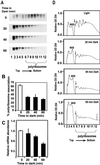
Ferredoxin-1 (Fed-1) mRNA contains an internal light response element (iLRE) that destabilizes mRNA when light-grown plants are placed in darkness. mRNAs containing this element dissociate from polyribosomes in the leaves of transgenic tobacco (Nicotiana tabacum) plants transferred to the dark for 2 d. Here, we report in vivo labeling experiments with a chloramphenicol acetyl transferase mRNA fused to the Fed-1 iLRE. Our data indicate that the Fed-1 iLRE mediates a rapid decline in translational efficiency and that iLRE-containing mRNAs dissociate from polyribosomes within 20 min after plants are transferred to darkness. Both events occur before the decline in mRNA abundance, and polyribosome association is rapidly reversible if plants are re-illuminated. These observations support a model in which Fed-1 mRNA in illuminated leaves is stabilized by its association with polyribosomes, and/or by translation. In darkness a large portion of the mRNA dissociates from polyribosomes and is subsequently degraded. We also show that a significant portion of total tobacco leaf mRNA is shifted from polyribosomal to non-polyribosomal fractions after 20 min in the dark, indicating that translation of other mRNAs is also rapidly down-regulated in response to darkness. This class includes some, but not all, cytoplasmic mRNAs encoding proteins involved in photosynthesis.
Figures


Similar articles
-
The Fed-1 (CAUU)4 element is a 5' UTR dark-responsive mRNA instability element that functions independently of dark-induced polyribosome dissociation.Plant Mol Biol. 2004 Nov;56(5):761-73. doi: 10.1007/s11103-004-5109-8. Epub 2005 Mar 24. Plant Mol Biol. 2004. PMID: 15803413
-
Light-regulated changes in abundance and polyribosome association of ferredoxin mRNA are dependent on photosynthesis.Plant Cell. 1997 Dec;9(12):2291-300. doi: 10.1105/tpc.9.12.2291. Plant Cell. 1997. PMID: 9437868 Free PMC article.
-
Light control of nuclear gene mRNA abundance and translation in tobacco.Plant Physiol. 2003 Dec;133(4):1979-90. doi: 10.1104/pp.103.029686. Plant Physiol. 2003. PMID: 14681536 Free PMC article.
-
Light regulation of Fed-1 mRNA requires an element in the 5' untranslated region and correlates with differential polyribosome association.Plant Cell. 1998 Mar;10(3):475-84. doi: 10.1105/tpc.10.3.475. Plant Cell. 1998. PMID: 9501119 Free PMC article.
-
Premature termination codons destabilize ferredoxin-1 mRNA when ferredoxin-1 is translated.Plant J. 2000 Mar;21(6):563-9. doi: 10.1046/j.1365-313x.2000.00705.x. Plant J. 2000. PMID: 10758507
Cited by
-
Mode of translational activation of the catalase (cat1) mRNA of rye leaves (Secale cereale L.) and its control through blue light and reactive oxygen.Planta. 2006 Mar;223(4):835-46. doi: 10.1007/s00425-005-0125-8. Epub 2006 Feb 23. Planta. 2006. PMID: 16341707
-
The Fed-1 (CAUU)4 element is a 5' UTR dark-responsive mRNA instability element that functions independently of dark-induced polyribosome dissociation.Plant Mol Biol. 2004 Nov;56(5):761-73. doi: 10.1007/s11103-004-5109-8. Epub 2005 Mar 24. Plant Mol Biol. 2004. PMID: 15803413
-
The early dark-response in Arabidopsis thaliana revealed by cDNA microarray analysis.Plant Mol Biol. 2006 Feb;60(3):321-42. doi: 10.1007/s11103-005-4211-x. Plant Mol Biol. 2006. PMID: 16514558
-
Translational Regulation of Cytoplasmic mRNAs.Arabidopsis Book. 2013 Jul 18;11:e0165. doi: 10.1199/tab.0165. Print 2013. Arabidopsis Book. 2013. PMID: 23908601 Free PMC article.
-
Carotenogenesis Is Regulated by 5'UTR-Mediated Translation of Phytoene Synthase Splice Variants.Plant Physiol. 2016 Dec;172(4):2314-2326. doi: 10.1104/pp.16.01262. Epub 2016 Oct 11. Plant Physiol. 2016. PMID: 27729470 Free PMC article.
References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Curr Protocols Mol Biol. 1998;2:10.16.11–10.16.13.
-
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. - PubMed
-
- Bovy A, Van den Berg C, De Vrieze G, Thompson WF, Weisbeek P, Smeekens S. Light-regulated expression of the Arabidopsis thaliana ferredoxin gene requires sequences upstream and downstream of the transcription initiation site. Plant Mol Biol. 1995;27:27–39. - PubMed
-
- Caspar T, Quail PH. Promoter and leader regions involved in the expression of the Arabidopsis ferredoxin A gene. Plant J. 1993;3:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

