Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem
- PMID: 11161035
- PMCID: PMC64879
- DOI: 10.1104/pp.125.2.779
Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem
Abstract
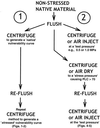
Although cavitation and refilling cycles could be common in plants, it is unknown whether these cycles weaken the cavitation resistance of xylem. Stem or petiole segments were tested for cavitation resistance before and after a controlled cavitation-refilling cycle. Cavitation was induced by centrifugation, air drying of shoots, or soil drought. Except for droughted plants, material was not significantly water stressed prior to collection. Cavitation resistance was determined from "vulnerability curves" showing the percentage loss of conductivity versus xylem pressure. Two responses were observed. "Resilient" xylem (Acer negundo and Alnus incana stems) showed no change in cavitation resistance after a cavitation-refilling cycle. In contrast, "weakened" xylem (Populus angustifolia, P. tremuloides, Helianthus annuus stems, and Aesculus hippocastanum petioles) showed considerable reduction in cavitation resistance. Weakening was observed whether cavitation was induced by centrifugation, air dehydration, or soil drought. Observations from H. annuus showed that weakening was proportional to the embolism induced by stress. Air injection experiments indicated that the weakened response was a result of an increase in the leakiness of the vascular system to air seeding. The increased air permeability in weakened xylem could result from rupture or loosening of the cellulosic mesh of interconduit pit membranes during the water stress and cavitation treatment.
Figures





References
-
- Alder NN, Pockman WT, Sperry JS, Nuismer S. Use of centrifugal force in the study of xylem cavitation. J Exp Bot. 1997;48:665–674.
-
- Alder NN, Sperry JS, Pockman WT. Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia. 1996;105:293–301. - PubMed
-
- Crombie DS, Hipkins MF, Milburn JA. Gas penetration of pit membranes in the xylem of Rhododendron and other species. Planta. 1985;163:27–33. - PubMed
-
- Dawson TE, Ehleringer JR. Gender specific physiology, carbon isotope discrimination, and habitat distribution in boxelder (Acer negundo) Ecology. 1993;74:798–815.
-
- Dina SD. An evaluation of physiological response to water stress as a factor influencing the distribution of six woody species in Red Butte Canyon, Utah. PhD Thesis. Salt Lake City: University of Utah; 1970.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

