A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs
- PMID: 11161039
- PMCID: PMC64883
- DOI: 10.1104/pp.125.2.818
A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs
Abstract
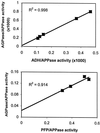
The occurrence of an extra-plastidial isoform of ADP-glucose (Glc) pyrophosphorylase (AGPase) among starch-storing organs was investigated in two ways. First, the possibility that an extra-plastidial isoform arose during the domestication of cereals was studied by comparing the intracellular distribution of enzyme activity and protein in developing endosperm of noncultivated Hordeum species with that previously reported for cultivated barley (Hordeum vulgare). As in cultivated barley, the AGPase of H. vulgare subsp. spontaneum and Hordeum murinum endosperm is accounted for by a major extra-plastidial and a minor plastidial isoform. Second, the ratio of ADP-Glc to UDP-Glc was used as an indication of the intracellular location of the AGPase activity in a wide range of starch-synthesizing organs. The ratio is expected to be high in organs in which UDP-Glc and ADP-Glc are synthesized primarily in the cytosol, because the reactions catalyzed by AGPase and UDP-Glc pyrophosphorylase will be coupled and close to equilibrium. This study revealed that ADP-Glc contents and the ratio of ADP-Glc to UDP-Glc were higher in developing graminaceous endosperms than in any other starch-storing organs. Taken as a whole the results indicate that an extra-plastidial AGPase is important in ADP-Glc synthesis in graminaceous endosperms, but not in other starch-storing organs.
Figures

References
-
- ap Rees T. Pathways of carbohydrate breakdown in plants. In: Northcote DH, editor. MTP International Review of Science: Biochemistry, Series One. Vol. 11. London: Butterworths; 1974. pp. 89–129.
-
- ap Rees T. Hexose phosphate metabolism by non-photosynthetic tissues of higher plants. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. New York: Academic Press; 1988. pp. 1–33.
-
- ap Rees T. Where do plants make ADPglucose? In: Pontis HG, Salerno GL, Echeverria EJ, editors. Sucrose: Metabolism, Biochemistry, Physiology and Molecular Biology. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 143–155.
-
- ap Rees T, Leja M, Macdonald FD, Green JH. Nucleotide sugars and starch synthesis in the spadix of Arum maculatum and suspension cultures of Glycine max. Phytochemistry. 1984;23:2463–2468.
-
- Bhattacharyya MK, Smith AM, Ellis THN, Hedley C, Martin C. The wrinkled seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch branching enzyme. Cell. 1990;60:115–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

