Pharmacodynamic models for the cardiovascular effects of moxonidine in patients with congestive heart failure
- PMID: 11167663
- PMCID: PMC2014427
- DOI: 10.1046/j.1365-2125.2001.01320.x
Pharmacodynamic models for the cardiovascular effects of moxonidine in patients with congestive heart failure
Abstract
Aims: To assess the pharmacodynamics of moxonidine in patients with functional NYHA Class II-III congestive heart failure (CHF).
Methods: A parallel population pharmacokinetic/pharmacodynamic (PK/PD) analysis was performed to assess the effect of moxonidine (0.1, 0.2, 0.3 mg twice daily) and placebo treatment on plasma noradrenaline (NA) levels, standing systolic blood pressure (SBP), and heart rate (HR) over 12 weeks in 97 patients with CHF using a parallel group design with dose escalation. A sequential analysis was also developed, where the relative changes in NA concentration were related to both SBP and HR.
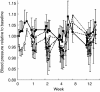
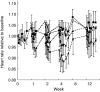
Results: In the parallel PD analysis, an effect delay was shown for all three end points (NA, SBP, and HR). An inhibitory Emax model was used to characterize the concentration-effect relationships. For SBP and HR, the EC50 value increased over time. For NA, there was a positive baseline drift over the 12 weeks; this was interpreted as disease progression. Moxonidine delayed this increase by 9.8 weeks. For SBP, there was a circadian pattern at baseline. In the sequential PD analysis, the relationship between the drug response (NA) and SBP or HR was best described by an inhibitory Emax model. No effect delays between the response and effects were found.
Conclusions: Effects of moxonidine on NA, SBP, and HR could be quantified by an effect compartment model in the presence of disease progression and circadian variations. Disease progression, as judged by increasing NA levels with time, was delayed by moxonidine. A direct relationship was found between NA and SBP/HR.
Figures


Similar articles
-
The effects of moxonidine, a novel imidazoline, on plasma norepinephrine in patients with congestive heart failure. Moxonidine Investigators.J Am Coll Cardiol. 2000 Feb;35(2):398-404. doi: 10.1016/s0735-1097(99)00565-3. J Am Coll Cardiol. 2000. PMID: 10676687 Clinical Trial.
-
The effects of chronic, sustained-release moxonidine therapy on clinical and neurohumoral status in patients with heart failure.Int J Cardiol. 2000 Sep 15;75(2-3):167-76; discussion 176-7. doi: 10.1016/s0167-5273(00)00319-3. Int J Cardiol. 2000. PMID: 11077130 Clinical Trial.
-
Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure.Circulation. 2002 Apr 16;105(15):1797-803. doi: 10.1161/01.cir.0000014212.04920.62. Circulation. 2002. PMID: 11956122 Clinical Trial.
-
The use of moxonidine in the treatment of hypertension.J Hypertens Suppl. 1997 Jan;15(1):S47-55. doi: 10.1097/00004872-199715011-00007. J Hypertens Suppl. 1997. PMID: 9050986 Review.
-
Moxonidine: some controversy.Expert Opin Pharmacother. 2001 Feb;2(2):337-50. doi: 10.1517/14656566.2.2.337. Expert Opin Pharmacother. 2001. PMID: 11336590 Review.
Cited by
-
Differential analysis of transient increases of serum cTnI in response to handling in rats.Pharmacol Res Perspect. 2013 Dec;1(2):e00011. doi: 10.1002/prp2.11. Epub 2013 Dec 5. Pharmacol Res Perspect. 2013. PMID: 25505566 Free PMC article.
-
Modeling delayed drug effect using discrete-time nonlinear autoregressive models: a connection with indirect response models.J Pharmacokinet Pharmacodyn. 2011 Jun;38(3):353-67. doi: 10.1007/s10928-011-9197-1. Epub 2011 Mar 31. J Pharmacokinet Pharmacodyn. 2011. PMID: 21451962
-
Evaluation of bias, precision, robustness and runtime for estimation methods in NONMEM 7.J Pharmacokinet Pharmacodyn. 2014 Jun;41(3):223-38. doi: 10.1007/s10928-014-9359-z. Epub 2014 May 7. J Pharmacokinet Pharmacodyn. 2014. PMID: 24801864
-
Model-based decision making in early clinical development: minimizing the impact of a blood pressure adverse event.AAPS J. 2009 Mar;11(1):99-108. doi: 10.1208/s12248-009-9083-6. Epub 2009 Feb 6. AAPS J. 2009. PMID: 19199043 Free PMC article.
-
Modelling the dose-response relationship: the fair share of pharmacokinetic and pharmacodynamic information.Br J Clin Pharmacol. 2017 Jun;83(6):1240-1251. doi: 10.1111/bcp.13225. Epub 2017 Feb 14. Br J Clin Pharmacol. 2017. PMID: 28035697 Free PMC article.
References
-
- The Consensus trial study group. Effects of enalapril on mortality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. - PubMed
-
- The SOLVD investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. - PubMed
-
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. - PubMed
-
- Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. - PubMed
-
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol. 1982;49:1659–1666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

