Mapping of early and late human somatosensory evoked brain potentials to phasic galvanic painful stimulation
- PMID: 11170308
- PMCID: PMC6871996
- DOI: 10.1002/1097-0193(200103)12:3<168::aid-hbm1013>3.0.co;2-o
Mapping of early and late human somatosensory evoked brain potentials to phasic galvanic painful stimulation
Abstract
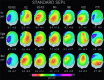
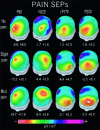
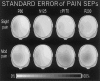
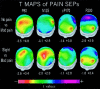
In the present study, we modeled the spatiotemporal evolution of human somatosensory evoked cortical potentials (SEPs) to brief median-nerve galvanic painful stimulation. SEPs were recorded (-50 to +250 ms) from 12 healthy subjects following nonpainful (reference), slight painful, and moderate painful stimulations (subjective scale). Laplacian transformation of scalp SEPs reduced head volume conduction effects and annulled electric reference influence. Typical SEP components to the galvanic nonpainful stimulation were contralateral frontal P20-N30-N60-N120-P170, central P22-P40, and parietal N20-P30-P60-P120 (N = negativity, P = positivity, number = latency in ms). These components were observed also with the painful stimulations, the N60, N120, P170 having a longer latency with the painful than nonpainful stimulations. Additional SEP components elicited by the painful stimulations were parietomedian P80 as well as central N125, P170 (cP170), and P200. These additional SEP components included the typical vertex negative-positive complex following transient painful stimulations. Latency of the SEP components exclusively elicited by painful stimulation is highly compatible with the involvement of A delta myelinated fibers/spinothalamic pathway. The topography of these components is in line with the response of both nociceptive medial and lateral systems including bilateral primary sensorimotor and anterior cingulate cortical areas. The role of attentive, affective, and motor aspects in the modulation of the reported SEP components merits investigation in future experiments.
Copyright 2001 Wiley-Liss, Inc.
Figures


References
-
- Allison T, McCarthy C, Wood S, Jones J (1991): Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114: 2465–2503. - PubMed
-
- Arendt‐Nielsen L (1994): Characteristics, detection, and modulation of laser‐evoked vertex potentials. Acta Anaesthesiol Scand Suppl 101: 7–44. - PubMed
-
- Arendt‐Nielsen L, Yamasaki H, Nielsen J, Naka D, Kakigi R (1999): Magnetoencephalographic responses to painful impact stimulation. Brain Res 839: 203–208. - PubMed
-
- Babiloni F, Babiloni C, Carducci F, Fattorini L, Onorati P, Urbano A (1996): Spline Laplacian estimate of EEG potentials over a realistic magnetic resonance‐constructed scalp surface model. Electroencephalogr Clin Neurophysiol 98: 363–373. - PubMed
-
- Babiloni F, Carducci F, Babiloni C, Urbano A (1998): Improved realistic Laplacian estimate of highly‐sampled EEG potentials by regularization techniques. Electroencephalogr Clin Neurophysiol 106: 336–343. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

