The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization
- PMID: 11171981
- PMCID: PMC29287
- DOI: 10.1073/pnas.98.4.1507
The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization
Abstract
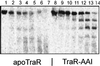
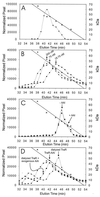
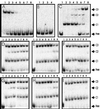
Complexes between the quorum-sensing regulator TraR and its inducing ligand autoinducer (AAI) are soluble in Escherichia coli, whereas apo-TraR is almost completely insoluble. Here we show that the lack of soluble TraR is due in large part to rapid proteolysis, inasmuch as apo-TraR accumulated to high levels in an E. coli strain deficient in Clp and Lon proteases. In pulse labeling experiments, AAI protected TraR against proteolysis only when it was added before the radiolabel. This observation indicates that TraR proteins can productively bind AAI only during their own synthesis on polysomes, whereas fully synthesized apo-TraR proteins are not functional AAI receptors. Purified apo-TraR was rapidly degraded by trypsin to oligopeptides, whereas TraR-AAI complexes were more resistant to trypsin and were cleaved at discrete interdomain linkers, indicating that TraR requires AAI to attain its mature tertiary structure. TraR-AAI complexes eluted from a gel filtration column as dimers and bound DNA as dimers. In contrast, apo-TraR was monomeric, and incubation with AAI under a variety of conditions did not cause dimerization. We conclude that AAI is critical for the folding of nascent TraR protein into its mature tertiary structure and that full-length apo-TraR cannot productively bind AAI and is consequently targeted for rapid proteolysis.
Figures


References
-
- Schleif R. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1300–1309.
-
- Choy H, Adhya S. In: in Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1287–1299.
-
- Hoch J A, Silhavy T J. Two-Component Signal Transduction. Washington, DC: Am. Soc. Microbiol.; 1995.
-
- Vierstra R D. Plant Mol Biol. 1996;32:275–302. - PubMed
-
- Dunny G M, Winans S C, editors. Cell–Cell Signaling in Bacteria. Washington, DC: Am. Soc. Microbiol.; 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

