Electrophysiological characterization of specific interactions between bacterial sensory rhodopsins and their transducers
- PMID: 11171989
- PMCID: PMC29295
- DOI: 10.1073/pnas.98.4.1555
Electrophysiological characterization of specific interactions between bacterial sensory rhodopsins and their transducers
Abstract
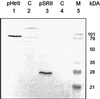
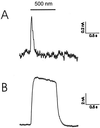
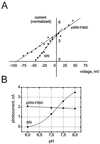
The halobacterial phototaxis receptors sensory rhodopsin I and II (SRI, SRII) enable the bacteria to seek optimal light conditions for ion pumping by bacteriorhodopsin and/or halorhodopsin. The incoming signal is transferred across the plasma membrane by means of receptor-specific transducer proteins that bind tightly to their corresponding photoreceptors. To investigate the receptor/transducer interaction, advantage is taken of the observation that both SRI and SRII can function as proton pumps. SRI from Halobacterium salinarum, which triggers the positive phototaxis, the photophobic receptor SRII from Natronobacterium pharaonis (pSRII), as well as the mutant pSRII-F86D were expressed in Xenopus oocytes. Voltage-clamp studies confirm that SRI and pSRII function as light-driven, outwardly directed proton pumps with a much stronger voltage dependence than the ion pumps bacteriorhodopsin and halorhodopsin. Coexpression of SRI and pSRII-F86D with their corresponding transducers suppresses the proton transport, revealing a tight binding and specific interaction of the two proteins. These latter results may be exploited to further analyze the binding interaction of the photoreceptors with their downstream effectors.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

