Embryonic expression of the tumor-associated PAX3-FKHR fusion protein interferes with the developmental functions of Pax3
- PMID: 11171995
- PMCID: PMC29301
- DOI: 10.1073/pnas.98.4.1589
Embryonic expression of the tumor-associated PAX3-FKHR fusion protein interferes with the developmental functions of Pax3
Abstract
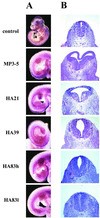
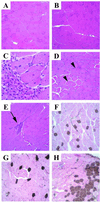
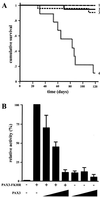
A unique chromosomal translocation involving the genes PAX3 and FKHR is characteristic of most human alveolar rhabdomyosarcomas. The resultant chimeric protein fuses the PAX3 DNA-binding domains to the transactivation domain of FKHR, suggesting that PAX3-FKHR exerts its role in alveolar rhabdomyosarcomas through dysregulation of PAX3-specific target genes. Here, we have produced transgenic mice in which PAX3-FKHR expression was driven by mouse Pax3 promoter/enhancer sequences. Five independent lines expressed PAX3-FKHR in the dorsal neural tube and lateral dermomyotome. Each line exhibited phenotypes that correlated with PAX3-FKHR expression levels and predominantly involved pigmentary disturbances of the abdomen, hindpaws, and tail, with additional neurological related alterations. Phenotypic severity could be increased by reducing Pax3 levels through matings with Pax3-defective Splotch mice, and interference between PAX3 and PAX3-FKHR was apparent in transcription reporter assays. These data suggest that the tumor-associated PAX3-FKHR fusion protein interferes with normal Pax3 developmental functions as a prelude to transformation.
Figures



References
-
- Kagan J, Croce C M. Ann Oncol. 1991;2:9–21. - PubMed
-
- Rabbitts T H. Nature (London) 1994;372:143–149. - PubMed
-
- Turc-Carel C, Lizard-Nacol S, Justrabo E, Favrot M, Philip T, Tabone E. Cancer Genet Cytogenet. 1986;19:361–362. - PubMed
-
- Douglass E C, Valentine M, Etcubanas E, Parham D, Webber B L, Houghton P J, Green A A. Cytogenet Cell Genet. 1987;45:148–155. - PubMed
-
- Galili N, Davis R J, Fredericks W J, Mukhopadhyay S, Rauscher F J, III, Emanuel B S, Rovera G, Barr F G. Nat Genet. 1993;5:230–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

