The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development
- PMID: 11172005
- PMCID: PMC29311
- DOI: 10.1073/pnas.98.4.1649
The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development
Abstract
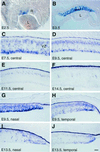
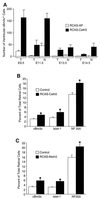
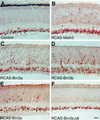
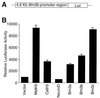
During retinogenesis, the Xenopus basic helix-loop-helix transcription factor Xath5 has been shown to promote a ganglion cell fate. In the developing mouse and chicken retinas, gene targeting and overexpression studies have demonstrated critical roles for the Brn3 POU domain transcription factor genes in the promotion of ganglion cell differentiation. However, the genetic relationship between Ath5 and Brn3 genes is unknown. To understand the genetic regulatory network(s) that controls retinal ganglion cell development, we analyzed the relationship between Ath5 and Brn3 genes by using a gain-of-function approach in the chicken embryo. We found that during retinogenesis, the chicken Ath5 gene (Cath5) is expressed in retinal progenitors and in differentiating ganglion cells but is absent in terminally differentiated ganglion cells. Forced expression of both Cath5 and the mouse Ath5 gene (Math5) in retinal progenitors activates the expression of cBrn3c following central-to-peripheral and temporal-to-nasal gradients. As a result, similar to the Xath5 protein, both Cath5 and Math5 proteins have the ability to promote the development of ganglion cells. Moreover, we found that forced expression of all three Brn3 genes also can stimulate the expression of cBrn3c. We further found that Ath5 and Brn3 proteins are capable of transactivating a Brn3b promoter. Thus, these data suggest that the expression of cBrn3c in the chicken and Brn3b in the mouse is initially activated by Ath5 factors in newly generated ganglion cells and later maintained by a feedback loop of Brn3 factors in the differentiated ganglion cells.
Figures

Similar articles
-
All Brn3 genes can promote retinal ganglion cell differentiation in the chick.Development. 2000 Aug;127(15):3237-47. doi: 10.1242/dev.127.15.3237. Development. 2000. PMID: 10887080
-
Identification of an N-terminal transcriptional activation domain within Brn3b/POU4f2.Differentiation. 2005 Feb;73(1):18-27. doi: 10.1111/j.1432-0436.2005.07301004.x. Differentiation. 2005. PMID: 15733064
-
Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth.Development. 2002 Jan;129(2):467-77. doi: 10.1242/dev.129.2.467. Development. 2002. PMID: 11807038
-
The role of basic helix-loop-helix genes in vertebrate retinogenesis.Semin Cell Dev Biol. 2001 Dec;12(6):491-8. doi: 10.1006/scdb.2001.0273. Semin Cell Dev Biol. 2001. PMID: 11735385 Review.
-
A gene regulatory hierarchy for retinal ganglion cell specification and differentiation.Semin Cell Dev Biol. 2004 Feb;15(1):115-23. doi: 10.1016/j.semcdb.2003.09.009. Semin Cell Dev Biol. 2004. PMID: 15036214 Review.
Cited by
-
Pushing the envelope of retinal ganglion cell genesis: context dependent function of Math5 (Atoh7).Dev Biol. 2012 Aug 15;368(2):214-30. doi: 10.1016/j.ydbio.2012.05.005. Epub 2012 May 15. Dev Biol. 2012. PMID: 22609278 Free PMC article.
-
A role of ath5 in inducing neuroD and the photoreceptor pathway.J Neurosci. 2004 Aug 11;24(32):7150-8. doi: 10.1523/JNEUROSCI.2266-04.2004. J Neurosci. 2004. PMID: 15306648 Free PMC article.
-
Gene expression in the developing mouse retina by EST sequencing and microarray analysis.Nucleic Acids Res. 2001 Dec 15;29(24):4983-93. doi: 10.1093/nar/29.24.4983. Nucleic Acids Res. 2001. PMID: 11812828 Free PMC article.
-
Common and divergent gene regulatory networks control injury-induced and developmental neurogenesis in zebrafish retina.Res Sq [Preprint]. 2023 Sep 18:rs.3.rs-3294233. doi: 10.21203/rs.3.rs-3294233/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Dec 20;14(1):8477. doi: 10.1038/s41467-023-44142-w. PMID: 37790324 Free PMC article. Updated. Preprint.
-
Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron specification and axonal projection into the spinal cord.Dev Biol. 2012 Apr 15;364(2):114-27. doi: 10.1016/j.ydbio.2012.01.021. Epub 2012 Feb 3. Dev Biol. 2012. PMID: 22326227 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

