RAD50 function is essential for telomere maintenance in Arabidopsis
- PMID: 11172016
- PMCID: PMC29322
- DOI: 10.1073/pnas.98.4.1711
RAD50 function is essential for telomere maintenance in Arabidopsis
Abstract
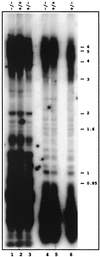
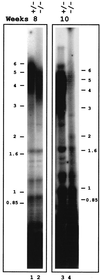
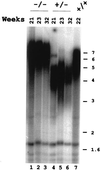
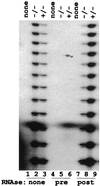
We have identified and characterized an Arabidopsis thaliana rad50 mutant plant containing a T-DNA insertion in the AtRAD50 gene and showing both meiotic and DNA repair defects. We report here that rad50/rad50 mutant cells show a progressive shortening of telomeric DNA relative to heterozygous rad50/RAD50 controls and that the mutant cell population rapidly enters a crisis, with the majority of the cells dying. Surviving rad50 mutant cells have longer telomeres than wild-type cells, indicating the existence in plants of an alternative RAD50-independent mechanism for telomere maintenance. These results report the role of a protein essential for double-strand break repair in telomere maintenance in higher eukaryotes.
Figures

Similar articles
-
Disruption of the Arabidopsis RAD50 gene leads to plant sterility and MMS sensitivity.Plant J. 2001 Jan;25(1):31-41. doi: 10.1046/j.1365-313x.2001.00928.x. Plant J. 2001. PMID: 11169180
-
Alteration of telomeric sequences and senescence caused by mutations in RAD50 of Saccharomyces cerevisiae.Genes Cells. 1997 Jul;2(7):443-55. doi: 10.1046/j.1365-2443.1997.1330331.x. Genes Cells. 1997. PMID: 9366550
-
RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase.Genetics. 1999 May;152(1):143-52. doi: 10.1093/genetics/152.1.143. Genetics. 1999. PMID: 10224249 Free PMC article.
-
[Functional regulation of the Mre11/Rad50/Xrs2 complex in the processes of recombination and DSB repair].Tanpakushitsu Kakusan Koso. 2001 Jun;46(8 Suppl):1030-7. Tanpakushitsu Kakusan Koso. 2001. PMID: 11436290 Review. Japanese. No abstract available.
-
The Mre11 complex: at the crossroads of dna repair and checkpoint signalling.Nat Rev Mol Cell Biol. 2002 May;3(5):317-27. doi: 10.1038/nrm805. Nat Rev Mol Cell Biol. 2002. PMID: 11988766 Review.
Cited by
-
Suppression of RICE TELOMERE BINDING PROTEIN 1 results in severe and gradual developmental defects accompanied by genome instability in rice.Plant Cell. 2007 Jun;19(6):1770-81. doi: 10.1105/tpc.107.051953. Epub 2007 Jun 22. Plant Cell. 2007. PMID: 17586654 Free PMC article.
-
Involvement of the Arabidopsis SWI2/SNF2 chromatin remodeling gene family in DNA damage response and recombination.Genetics. 2006 Jun;173(2):985-94. doi: 10.1534/genetics.105.051664. Epub 2006 Mar 17. Genetics. 2006. PMID: 16547115 Free PMC article.
-
DNA damage response in plants: conserved and variable response compared to animals.Biology (Basel). 2013 Nov 21;2(4):1338-56. doi: 10.3390/biology2041338. Biology (Basel). 2013. PMID: 24833228 Free PMC article.
-
DNA repair and recombination functions in Arabidopsis telomere maintenance.Chromosome Res. 2005;13(5):481-91. doi: 10.1007/s10577-005-0995-4. Chromosome Res. 2005. PMID: 16132813 Review.
-
Meiotic localization of Mre11 and Rad50 in wild type, spo11-1, and MRN complex mutants of Coprinus cinereus.Chromosoma. 2009 Aug;118(4):471-86. doi: 10.1007/s00412-009-0209-5. Epub 2009 Apr 26. Chromosoma. 2009. PMID: 19396455
References
-
- Zakian V A. Annu Rev Genet. 1996;30:141–172. - PubMed
-
- Greider C W. Annu Rev Biochem. 1996;65:337–365. - PubMed
-
- McKnight T D, Fitzgerald M S, Shippen D E. Biochemistry. 1997;62:1224–1231. - PubMed
-
- Richards E J, Ausubel F M. Cell. 1988;53:127–136. - PubMed
-
- Greider C W, Blackburn E H. Cell. 1985;43:405–413. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

