Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells
- PMID: 11172024
- PMCID: PMC29330
- DOI: 10.1073/pnas.98.4.1757
Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells
Abstract
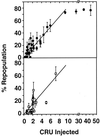
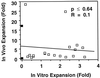
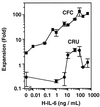
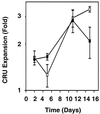
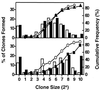
Previous studies have demonstrated hematopoietic stem cell amplification in vitro after the activation of three cell-surface receptors: flt3/flk2, c-kit, and gp130. We now show flt3-ligand and Steel factor alone will stimulate >85% of c-kit(+)Sca-1(+)lin(-) adult mouse bone marrow cells to proliferate in single-cell serum-free cultures, but concomitant retention of their stem cell activity requires additional exposure to a ligand that will activate gp130. Moreover, this response is restricted to a narrow range of gp130-activating ligand concentrations, above and below which hematopoietic stem cell activity is lost. These findings indicate a unique contribution of gp130 signaling to the maintenance of hematopoietic stem cell function when these cells are stimulated to divide with additional differential effects dictated by the intensity of gp130 activation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

