A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V
- PMID: 11172035
- PMCID: PMC29341
- DOI: 10.1073/pnas.98.4.1823
A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V
Abstract
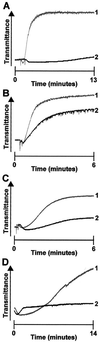
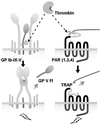
Glycoprotein (GP) V is a major substrate cleaved by the protease thrombin during thrombin-induced platelet activation. Previous analysis of platelets from GP V-null mice suggested a role for GP V as a negative modulator of platelet activation by thrombin. We now report the mechanism by which thrombin activates GP V -/- platelets. We show that proteolytically inactive forms of thrombin induce robust stimulatory responses in GP V null mouse platelets, via the platelet GP Ib--IX--V complex. Because proteolytically inactive thrombin can activate wild-type mouse and human platelets after treatment with thrombin to cleave GP V, this mechanism is involved in thrombin-induced platelet aggregation. Platelet activation through GP Ib-IX depends on ADP secretion, and specific inhibitors demonstrate that the recently cloned P2Y(12) ADP receptor (G(i)-coupled ADP receptor) is involved in this pathway, and that the P2Y(1) receptor (G(q)-coupled ADP receptor) may play a less significant role. Thrombosis was generated in GP V null mice only in response to catalytically inactive thrombin, whereas thrombosis occurred in both genotypes (wild type and GP V null) in response to active thrombin. These data support a thrombin receptor function for the platelet membrane GP Ib--IX--V complex, and describe a novel thrombin signaling mechanism involving an initiating proteolytic event followed by stimulation of the GP Ib--IX via thrombin acting as a ligand, resulting in platelet activation.
Figures



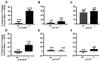
Comment in
-
A new "kid" on the platelet thrombin receptor "block": glycoprotein Ib-IX-V.Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1330-1. doi: 10.1073/pnas.98.4.1330. Proc Natl Acad Sci U S A. 2001. PMID: 11171948 Free PMC article. No abstract available.
References
-
- Lopez J A, Andrews R K, Afshar-Kharghan V, Berndt M C. Blood. 1998;91:4397–4418. - PubMed
-
- Ruggeri Z M, Dent J A, Saldivar E. Blood. 1999;94:172–178. - PubMed
-
- Calverley D C, Kavanagh T J, Roth G J. Blood. 1998;91:1295–1303. - PubMed
-
- Andrews R K, Harris S J, McNally T, Berndt M C. Biochemistry. 1998;37:638–647. - PubMed
-
- Asazuma N, Ozaki Y, Satoh K, Yatomi Y, Handa M, Fujimura Y, Miura S, Kume S. Blood. 1997;90:4789–4798. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

