Integrin activation controls metastasis in human breast cancer
- PMID: 11172040
- PMCID: PMC29346
- DOI: 10.1073/pnas.98.4.1853
Integrin activation controls metastasis in human breast cancer
Abstract
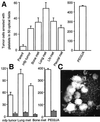
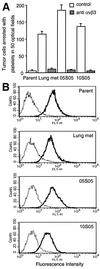
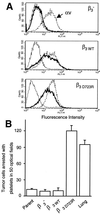
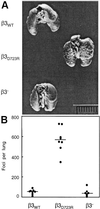
Metastasis is the primary cause of death in human breast cancer. Metastasis to bone, lungs, liver, and brain involves dissemination of breast cancer cells via the bloodstream and requires adhesion within the vasculature. Blood cell adhesion within the vasculature depends on integrins, a family of transmembrane adhesion receptors, and is regulated by integrin activation. Here we show that integrin alpha v beta 3 supports breast cancer cell attachment under blood flow conditions in an activation-dependent manner. Integrin alpha v beta 3 was found in two distinct functional states in human breast cancer cells. The activated, but not the nonactivated, state supported tumor cell arrest during blood flow through interaction with platelets. Importantly, activated alpha v beta 3 was expressed by freshly isolated metastatic human breast cancer cells and variants of the MDA-MB 435 human breast cancer cell line, derived from mammary fat pad tumors or distant metastases in severe combined immunodeficient mice. Expression of constitutively activated mutant alpha v beta 3(D723R), but not alpha v beta 3(WT), in MDA-MB 435 cells strongly promoted metastasis in the mouse model. Thus breast cancer cells can exhibit a platelet-interactive and metastatic phenotype that is controlled by the activation of integrin alpha v beta 3. Consequently, alterations within tumors that lead to the aberrant control of integrin activation are expected to adversely affect the course of human breast cancer.
Figures

References
-
- Price J T, Bonovich M T, Kohn E C. Crit Rev Biochem Mol Biol. 1997;32:175–253. - PubMed
-
- Ruoslahti E. Adv Cancer Res. 1999;76:1–20. - PubMed
-
- Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. - PubMed
-
- Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. - PubMed
-
- Albelda S M, Mette S A, Elder D E, Stewart R, Damjanovich L, Herlyn M, Buck C A. Cancer Res. 1990;50:6757–6764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

