Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts
- PMID: 11178102
- PMCID: PMC29065
- DOI: 10.1186/1471-2121-1-4
Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts
Abstract
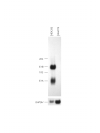
Background: The hepatic stellate cell is the primary cell type responsible for the excessive formation and deposition of connective tissue elements during the development of hepatic fibrosis in chronically injured liver. Culturing quiescent hepatic stellate cells on plastic causes spontaneous activation leading to a myofibroblastic phenotype similar to that seen in vivo. This provides a simple model system for studying activation and transdifferentiation of these cells. The introduction of exogenous DNA into these cells is discussed controversially mainly due to the lack of systematic analysis. Therefore, we examined comparatively five nonviral, lipid-mediated gene transfer methods and adenoviral based infection, as potential tools for efficient delivery of DNA to rat hepatic stellate cells and their transdifferentiated counterpart, i.e. myofibroblasts. Transfection conditions were determined using enhanced green fluorescent protein as a reporter expressed under the transcriptional control of the human cytomegalovirus immediate early gene 1 promoter/enhancer.
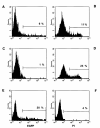
Results: With the use of chemically enhanced transfection methods, the highest relative efficiency was obtained with FuGENE6 gene mediated DNA transfer. Quantitative evaluation of representative transfection experiments by flow cytometry revealed that approximately 6% of the rat hepatic stellate cells were transfected. None of the transfection methods tested was able to mediate gene delivery to rat myofibroblasts. To analyze if rat hepatic stellate cells and myofibroblasts are susceptible to adenoviral infection, we have inserted the transgenic expression cassette into a recombinant adenoviral type 5 genome as replacement for the E1 region. Viral particles of this replication-deficient Ad5-based reporter are able to infect 100% of rat hepatic stellate cells and myofibroblasts, respectively.
Conclusions: Our results indicate that FuGENE6-based methods may be optimized sufficiently to offer a feasible approach for gene transfer into rat hepatic stellate cells. The data further demonstrate that adenoviral mediated transfer is a promising approach for gene delivery to these hepatic cells.
Figures


Similar articles
-
Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function?J Cell Mol Med. 2007 Jul-Aug;11(4):704-22. doi: 10.1111/j.1582-4934.2007.00060.x. J Cell Mol Med. 2007. PMID: 17760834 Free PMC article. Review.
-
A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture.Hepatology. 1999 Mar;29(3):858-67. doi: 10.1002/hep.510290328. Hepatology. 1999. PMID: 10051490
-
p53 adenoviral vector (Ad-CMV-p53) induced prostatic growth inhibition of primary cultures of human prostate and an experimental rat model.J Gene Med. 2000 Nov-Dec;2(6):426-32. doi: 10.1002/1521-2254(200011/12)2:6<426::AID-JGM140>3.0.CO;2-2. J Gene Med. 2000. PMID: 11199263
-
Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts.Hepatology. 2000 May;31(5):1094-106. doi: 10.1053/he.2000.6126. Hepatology. 2000. PMID: 10796885
-
Cytoglobin as a Marker of Hepatic Stellate Cell-derived Myofibroblasts.Front Physiol. 2015 Nov 13;6:329. doi: 10.3389/fphys.2015.00329. eCollection 2015. Front Physiol. 2015. PMID: 26617531 Free PMC article. Review.
Cited by
-
Analysis of novel nonviral gene transfer systems for gene delivery to cells of the musculoskeletal system.Mol Biotechnol. 2008 Feb;38(2):137-44. doi: 10.1007/s12033-007-0071-8. Epub 2007 Oct 17. Mol Biotechnol. 2008. PMID: 18219593
-
Connective tissue growth factor reacts as an IL-6/STAT3-regulated hepatic negative acute phase protein.World J Gastroenterol. 2011 Jan 14;17(2):151-63. doi: 10.3748/wjg.v17.i2.151. World J Gastroenterol. 2011. PMID: 21245987 Free PMC article.
-
Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function?J Cell Mol Med. 2007 Jul-Aug;11(4):704-22. doi: 10.1111/j.1582-4934.2007.00060.x. J Cell Mol Med. 2007. PMID: 17760834 Free PMC article. Review.
-
Effects of pharmacological serum from normal and liver fibrotic rats on HSCs.World J Gastroenterol. 2005 Apr 28;11(16):2444-9. doi: 10.3748/wjg.v11.i16.2444. World J Gastroenterol. 2005. PMID: 15832415 Free PMC article.
-
Endoglin Trafficking/Exosomal Targeting in Liver Cells Depends on N-Glycosylation.Cells. 2019 Aug 28;8(9):997. doi: 10.3390/cells8090997. Cells. 2019. PMID: 31466384 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

