Genetic modification of glaucoma associated phenotypes between AKXD-28/Ty and DBA/2J mice
- PMID: 11178107
- PMCID: PMC29081
- DOI: 10.1186/1471-2156-2-1
Genetic modification of glaucoma associated phenotypes between AKXD-28/Ty and DBA/2J mice
Abstract
Background: Glaucoma is a common disease but its molecular etiology is poorly understood. It involves retinal ganglion cell death and optic nerve damage that is often associated with elevated intraocular pressure. Identifying genes that modify glaucoma associated phenotypes is likely to provide insights to mechanisms of glaucoma. We previously reported glaucoma in DBA/2J mice caused by recessive alleles at two loci, isa and ipd, that cause iris stromal atrophy and iris pigment dispersion, respectively. A approach for identifying modifier genes is to study the effects of specific mutations in different mouse strains. When the phenotypic effect of a mutation is modified upon its introduction into a new strain, crosses between the parental strains can be used to identify modifier genes. The purpose of this study was to determine if the effects of the DBA/2J derived isa and ipd loci are modified in strain AKXD-28/Ty.
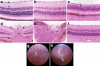
Results: AKXD-28/Ty mice develop glaucoma characterized by intraocular pressure elevation, retinal ganglion loss, and optic nerve excavation. In AKXD-28/Ty, isa causes an iris stromal atrophy phenotype as in DBA/2J. However, the iris pigment dispersion phenotype associated with ipd in DBA/2J does not occur in AKXD-28/Ty. Additionally, a greater severity and speed of retinal and optic nerve damage following intraocular pressure elevation in AKXD-28/Ty compared to DBA/2J mice suggests that AKXD-28/Ty is more susceptible to pressure-induced cell death.
Conclusions: The consequences of the ipd and isa mutations are modified in the AKXD-28/Ty background. These strains provide a resource for the identification of modifier genes that modulate pigment dispersion and susceptibility to pressure-induced cell death.
Figures



Similar articles
-
Cochlin and glaucoma: a mini-review.Vis Neurosci. 2005 Sep-Oct;22(5):605-13. doi: 10.1017/S0952523805225099. Vis Neurosci. 2005. PMID: 16332271 Free PMC article. Review.
-
Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice.Nat Genet. 1999 Apr;21(4):405-9. doi: 10.1038/7741. Nat Genet. 1999. PMID: 10192392
-
Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice.Nat Genet. 2002 Jan;30(1):81-5. doi: 10.1038/ng794. Epub 2001 Dec 17. Nat Genet. 2002. PMID: 11743578
-
Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice.Invest Ophthalmol Vis Sci. 1998 May;39(6):951-62. Invest Ophthalmol Vis Sci. 1998. PMID: 9579474
-
Mouse models of retinal ganglion cell death and glaucoma.Exp Eye Res. 2009 Apr;88(4):816-24. doi: 10.1016/j.exer.2008.12.002. Epub 2008 Dec 7. Exp Eye Res. 2009. PMID: 19105954 Free PMC article. Review.
Cited by
-
Quantitative trait loci on chromosome 1 for cataract and AMD-like retinopathy in senescence-accelerated OXYS rats.Aging (Albany NY). 2012 Jan;4(1):49-59. doi: 10.18632/aging.100427. Aging (Albany NY). 2012. PMID: 22300709 Free PMC article.
-
Cochlin and glaucoma: a mini-review.Vis Neurosci. 2005 Sep-Oct;22(5):605-13. doi: 10.1017/S0952523805225099. Vis Neurosci. 2005. PMID: 16332271 Free PMC article. Review.
-
YBR/EiJ mice: a new model of glaucoma caused by genes on chromosomes 4 and 17.Dis Model Mech. 2016 Aug 1;9(8):863-71. doi: 10.1242/dmm.024307. Epub 2016 Jun 9. Dis Model Mech. 2016. PMID: 27483353 Free PMC article.
-
Central corneal thickness does not correlate with TonoLab-measured IOP in several mouse strains with single transgenic mutations of matricellular proteins.Exp Eye Res. 2013 Oct;115:106-12. doi: 10.1016/j.exer.2013.06.017. Epub 2013 Jun 24. Exp Eye Res. 2013. PMID: 23806329 Free PMC article.
-
Genetic modifiers and oligogenic inheritance.Cold Spring Harb Perspect Med. 2015 Jun 1;5(6):a017145. doi: 10.1101/cshperspect.a017145. Cold Spring Harb Perspect Med. 2015. PMID: 26033081 Free PMC article. Review.
References
-
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. - PubMed
-
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. - PubMed
-
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. - PubMed
-
- Shields MB, Ritch R, Krupin T. Classifications of the Glaucomas. The Glaucomas Edited by Ritch R, Shields MB, Krupin T, vol. 2. pp. 717-725. St. Louis: Mosby; 1996. pp. 717–725.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

