A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies
- PMID: 11179018
- PMCID: PMC1274483
- DOI: 10.1086/318809
A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies
Abstract
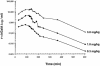
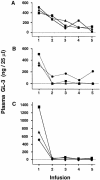
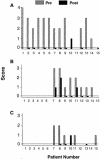
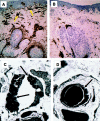
Fabry disease results from deficient alpha-galactosidase A (alpha-Gal A) activity and the pathologic accumulation of the globotriaosylceramide (GL-3) and related glycosphingolipids, primarily in vascular endothelial lysosomes. Treatment is currently palliative, and affected patients generally die in their 40s or 50s. Preclinical studies of recombinant human alpha-Gal A (r-halphaGalA) infusions in knockout mice demonstrated reduction of GL-3 in tissues and plasma, providing rationale for a phase 1/2 clinical trial. Here, we report a single-center, open-label, dose-ranging study of r-halphaGalA treatment in 15 patients, each of whom received five infusions at one of five dose regimens. Intravenously administered r-halphaGalA was cleared from the circulation in a dose-dependent manner, via both saturable and non-saturable pathways. Rapid and marked reductions in plasma and tissue GL-3 were observed biochemically, histologically, and/or ultrastructurally. Clearance of plasma GL-3 was dose-dependent. In patients with pre- and posttreatment biopsies, mean GL-3 content decreased 84% in liver (n=13), was markedly reduced in kidney in four of five patients, and after five doses was modestly lowered in the endomyocardium of four of seven patients. GL-3 deposits were cleared to near normal or were markedly reduced in the vascular endothelium of liver, skin, heart, and kidney, on the basis of light- and electron-microscopic evaluation. In addition, patients reported less pain, increased ability to sweat, and improved quality-of-life measures. Infusions were well tolerated; four patients experienced mild-to-moderate reactions, suggestive of hypersensitivity, that were managed conservatively. Of 15 patients, 8 (53%) developed IgG antibodies to r-halphaGalA; however, the antibodies were not neutralizing, as indicated by unchanged pharmacokinetic values for infusions 1 and 5. This study provides the basis for a phase 3 trial of enzyme-replacement therapy for Fabry disease.
Figures



References
Electronic-Database Information
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for Fabry disease [MIM 301500])
References
-
- Brady RO, Tallman JF, Johnson WG, Gal AE, Leahy WR, Quirk JM, Dekaban AS (1973) Replacement therapy for inherited enzyme deficiency: use of purified ceramidetrihexosidase in Fabry's disease. N Engl J Med 289:9–14 - PubMed
-
- Colombi A, Kostyal A, Bracher R, Gloor F, Mazzi R, Tholen H (1967) Angiokeratoma corporis diffusum: Fabry's disease. Helv Med Acta 34:67–83 - PubMed
-
- Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W (1973) Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes: α-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med 81:157–171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

