Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis
- PMID: 11179223
- PMCID: PMC145417
- DOI: 10.1093/emboj/20.4.792
Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis
Abstract
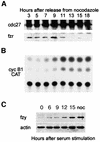
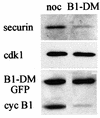
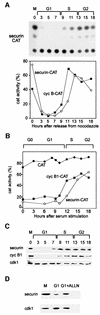
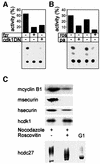
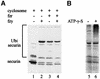
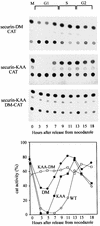
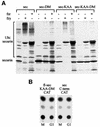
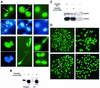
We have studied the ubiquitination and degradation patterns of the human securin/PTTG protein. We show that, in contrast to budding yeast pds1, securin degradation is catalyzed by both fzy (fizzy/cdc20) and fzr (fizzy-related/cdh1/hct1). Both fzy and fzr also induce the APC/C to ubiquitinate securin in vitro. Securin degradation is mediated by an RXXL destruction box and a KEN box, and is inhibited only when both sequences are mutated. Interestingly, the non-degradable securin mutant is also partially ubiquitinated by fzy and fzr in vitro. Expressing the non-degradable securin mutant in cells frequently resulted in incomplete chromatid separation and gave rise to daughter cells connected by a thin chromatin fiber, presumably of chromosomes that failed to split completely. Strikingly, the mutant securin did not prevent the majority of sister chromatids from separating completely, nor did it prevent mitotic cyclin degradation and cytokinesis. This phenotype, reminiscent of the fission yeast cut (cells untimely torn) phenotype, is reported here for the first time in mammals.
Figures
References
-
- Amon A., Irniger,S. and Nasmyth,K. (1994) Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell, 77, 1037–1050. - PubMed
-
- Antonio C., Ferby,I., Wilhelm,H., Jones,M., Karsenti,E., Nebreda,A. and Vernos,I. (2000) Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell, 102, 425–435. - PubMed
-
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1994) Current Protocols in Molecular Biology. John Wiley & Sons Inc., New York, NY.
-
- Ciosk R., Zachariae,W., Michaelis,C., Shevchenko,A., Mann,M. and Nasmyth,K. (1998) An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell, 93, 1067–1076. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

