Cell vacuolation caused by Vibrio cholerae hemolysin
- PMID: 11179335
- PMCID: PMC98064
- DOI: 10.1128/IAI.69.3.1613-1624.2001
Cell vacuolation caused by Vibrio cholerae hemolysin
Abstract
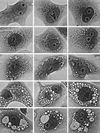
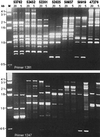
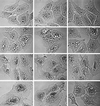
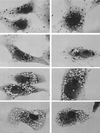
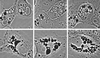
Non-O1 strains of Vibrio cholerae implicated in gastroenteritis and diarrhea generally lack virulence determinants such as cholera toxin that are characteristic of epidemic strains; the factors that contribute to their virulence are not understood. Here we report that at least one-third of diarrhea-associated nonepidemic V. cholerae strains from Mexico cause vacuolation of cultured Vero cells. Detailed analyses indicated that this vacuolation was related to that caused by aerolysin, a pore-forming toxin of Aeromonas; it involved primarily the endoplasmic reticulum at early times (approximately 1 to 4 h after exposure), and resulted in formation of large, acidic, endosome-like multivesicular vacuoles (probably autophagosomes) only at late times (approximately 16 h). In contrast to vacuolation caused by Helicobacter pylori VacA protein, that induced by V. cholerae was exacerbated by agents that block vacuolar proton pumping but not by endosome-targeted weak bases. It caused centripetal redistribution of endosomes, reflecting cytoplasmic alkalinization. The gene for V. cholerae vacuolating activity was cloned and was found to correspond to hlyA, the structural gene for hemolysin. HlyA protein is a pore-forming toxin that causes ion leakage and, ultimately, eukaryotic cell lysis. Thus, a distinct form of cell vacuolation precedes cytolysis at low doses of hemolysin. We propose that this vacuolation, in itself, contributes to the virulence of V. cholerae strains, perhaps by perturbing intracellular membrane trafficking or ion exchange in target cells and thereby affecting local intestinal inflammatory or other defense responses.
Figures


References
-
- Alm R A, Stroher U H, Manning P A. Extracellular proteins of Vibrio cholerae: nucleotide sequence of the structural gene (hlyA) for the haemolysin of the haemolytic El Tor strain O17 and characterization of the hlyA mutation in the non haemolytic classical strain 569B. Mol Microb. 1988;2:481–488. - PubMed
-
- Alm R A, Mayrhofer G, Kotlarski I, Manning P A. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine. 1991;9:588–594. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: Wiley Interscience; 1989.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

