Regulation of cell function by methionine oxidation and reduction
- PMID: 11179387
- PMCID: PMC2278441
- DOI: 10.1111/j.1469-7793.2001.0001j.x
Regulation of cell function by methionine oxidation and reduction
Abstract
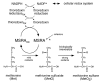
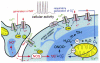
Reactive oxygen species (ROS) are generated during normal cellular activity and may exist in excess in some pathophysiological conditions, such as inflammation or reperfusion injury. These molecules oxidize a variety of cellular constituents, but sulfur-containing amino acid residues are especially susceptible. While reversible cysteine oxidation and reduction is part of well-established signalling systems, the oxidation and the enzymatically catalysed reduction of methionine is just emerging as a novel molecular mechanism for cellular regulation. Here we discuss how the oxidation of methionine to methionine sulfoxide in signalling proteins such as ion channels affects the function of these target proteins. Methionine sulfoxide reductase, which reduces methionine sulfoxide to methionine in a thioredoxin-dependent manner, is therefore not only an enzyme important for the repair of age- or degenerative disease-related protein modifications. It is also a potential missing link in the post-translational modification cycle involved in the specific oxidation and reduction of methionine residues in cellular signalling proteins, which may give rise to activity-dependent plastic changes in cellular excitability.
Figures
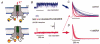
References
-
- Aldrich RW, Hoshi T, Zagotta WN. Differences in gating among amino-terminal variants of Shaker potassium channels. Cold Spring Harbor Symposia on Quantitative Biology. 1990;55:19–27. - PubMed
-
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. American Journal of Physiology. 1998;275:1283–1289. H. - PubMed
-
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. Journal of Biological Chemistry. 1997;272:20313–20316. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Reviews/Molecular Cell Biology. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

