Intracellular pathways regulating ciliary beating of rat brain ependymal cells
- PMID: 11179397
- PMCID: PMC2278437
- DOI: 10.1111/j.1469-7793.2001.0131j.x
Intracellular pathways regulating ciliary beating of rat brain ependymal cells
Abstract
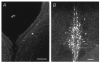
1. The mammalian brain ventricles are lined with ciliated ependymal cells. As yet little is known about the mechanisms by which neurotransmitters regulate cilia beat frequency (CBF). 2. Application of 5-HT to ependymal cells in cultured rat brainstem slices caused CBF to increase. 5-HT had an EC50 of 30 microM and at 100 microM attained a near-maximal CBF increase of 52.7 +/- 4.1 % (mean +/- s.d.) (n = 8). 3. Bathing slices in Ca2+-free solution markedly reduced the 5-HT-mediated increase in CBF. Fluorescence measurements revealed that 5-HT caused a marked transient elevation in cytosolic Ca2+ ([Ca2+]c) that then slowly decreased to a plateau level. Analysis showed that the [Ca2+]c transient was due to release of Ca2+ from inositol 1,4,5-trisphosphate (IP3)-sensitive stores; the plateau was probably due to extracellular Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels. 4. Application of ATP caused a sustained decrease in CBF. ATP had an EC50 of about 50 microM and 100 microM ATP resulted in a maximal 57.5 +/- 6.5 % (n = 12) decrease in CBF. The ATP-induced decrease in CBF was unaffected by lowering extracellular [Ca2+], and no changes in [Ca2+]c were observed. Exposure of ependymal cells to forskolin caused a decrease in CBF. Ciliated ependymal cells loaded with caged cAMP exhibited a 54.3 +/- 7.5 % (n = 9) decrease in CBF following uncaging. These results suggest that ATP reduces CBF by a Ca2+-independent cAMP-mediated pathway. 5. Application of 5-HT and adenosine-5'-O-3-thiotriphosphate (ATP-gamma-S) to acutely isolated ciliated ependymal cells resulted in CBF responses similar to those of ependymal cells in cultured slices suggesting that these neurotransmitters act directly on these cells. 6. The opposite response of ciliated ependymal cells to 5-HT and ATP provides a novel mechanism for their active involvement in central nervous system signalling.
Figures
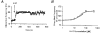





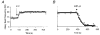

References
-
- Afzelius BA, Mossberg B. Immotile-cilia syndrome (primary ciliary dyskinesia), including Kartagener syndrome. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 3943–3954.
-
- Ben-Shimol Y, Dinstein I, Meisels A, Priel Z. Ciliary motion features from digitized video photography. Journal of Computer-Assisted Microscopy. 1991;3:103–116.
-
- Böhm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. Journal of Biological Chemistry. 1996;271:22003–22016. - PubMed
-
- Cathcart RS, Worthington WC. Ciliary movement in the rat cerebral ventricles: clearing action and directions of currents. Journal of Neuropathology and Experimental Neurology. 1964;23:609–618. - PubMed
-
- Chan-Paly V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Research. 1976;102:103–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

