Voluntary contraction impairs the refractory period of transmission in healthy human axons
- PMID: 11179409
- PMCID: PMC2278452
- DOI: 10.1111/j.1469-7793.2001.0265j.x
Voluntary contraction impairs the refractory period of transmission in healthy human axons
Abstract
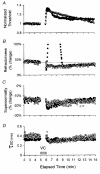
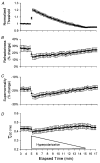
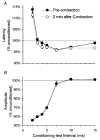
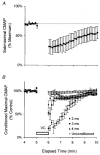
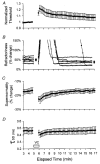
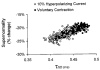
1. Voluntary contraction of a muscle causes substantial hyperpolarization of the active motor axons due to activation of the electrogenic Na+-K+ pump. The present study was undertaken to determine whether voluntary effort produces a significant impairment in impulse transmission in normal axons and whether mechanisms other than membrane hyperpolarization contribute to the changes in axonal excitability. 2. The compound muscle action potential (CMAP) was recorded after median nerve stimulation at the wrist using sub- and supramaximal stimuli, delivered singly and in pairs at conditioning-test intervals of 2-15 ms. Axonal excitability parameters (threshold, refractoriness, supernormality, and strength-duration time constant (tauSD)) were measured using threshold tracking. Impulse transmission was assessed using supramaximal stimuli. 3. Maximal voluntary contractions of the abductor pollicis brevis for 1 min produced a substantial increase in threshold, an increase in supernormality and a decrease in tauSD, all of which lasted approximately 10 min and indicate axonal hyperpolarization. However, immediately after the contraction there was an unexpected increase in refractoriness. The post-contraction increase in refractoriness could not be mimicked by an imposed ramp of hyperpolarization that produced changes in the other indices to an extent that was similar to voluntary contraction. 4. The contraction had relatively little effect on the size of the unconditioned maximal CMAP. However, there was failure of transmission of supramaximal conditioned volleys when the conditioning-test interval was short. 5. The relationships between axonal excitability and supernormality and tauSD following voluntary contraction differed significantly from those recorded during the hyperpolarization produced by DC current. It is argued that these differences probably result from extra-axonal K+ accumulation with the voluntary contraction but not with the DC polarization. I6. It is concluded that, following maximal voluntary contraction of a normal muscle, the refractory period of transmission is impaired distal to the stimulus site sufficient to cause transmission failure of the second of a pair of closely spaced impulses. The site of transmission failure is likely to be the terminal axon, presumably at branch points, possibly in the unmyelinated pre-terminal segment.
Figures


References
-
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor unit discharge rates in maximal voluntary contraction of three human muscles. Journal of Neurophysiology. 1983;50:1380–1392. - PubMed
-
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor nerves in vivo. Brain Research. 1988;462:354–358. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

