Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis
- PMID: 11179425
- PMCID: PMC30953
- DOI: 10.1091/mbc.12.2.421
Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis
Abstract
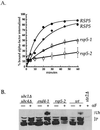
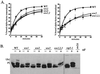
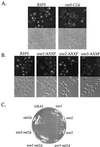
Yeast Rsp5p and its mammalian homologue, Nedd4, are hect domain ubiquitin-protein ligases (E3s) required for the ubiquitin-dependent endocytosis of plasma membrane proteins. Because ubiquitination is sufficient to induce internalization, E3-mediated ubiquitination is a key regulatory event in plasma membrane protein endocytosis. Rsp5p is an essential, multidomain protein containing an amino-terminal C2 domain, three WW protein-protein interaction domains, and a carboxy-terminal hect domain that carries E3 activity. In this study, we demonstrate that Rsp5p is peripherally associated with membranes and provide evidence that Rsp5p functions as part of a multimeric protein complex. We define the function of Rsp5p and its domains in the ubiquitin-dependent internalization of the yeast alpha-factor receptor, Ste2p. Temperature-sensitive rsp5 mutants were unable to ubiquitinate or to internalize Ste2p at the nonpermissive temperature. Deletion of the entire C2 domain had no effect on alpha-factor internalization; however, point mutations in any of the three WW domains impaired both receptor ubiquitination and internalization. These observations indicate that the WW domains play a role in the important regulatory event of selecting phosphorylated proteins as endocytic cargo. In addition, mutations in the C2 and WW1 domains had more severe defects on transport of fluid-phase markers to the vacuole than on receptor internalization, suggesting that Rsp5p functions at multiple steps in the endocytic pathway.
Figures




References
-
- Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. - PubMed
-
- Bedford MT, Sarbassova D, Xu J, Leder P, Yaffe MB. A novel Pro-Arg motif recognized by WW domains. J Biol Chem. 2000;275:10359–10369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

