Structural and functional analysis of horse cathelicidin peptides
- PMID: 11181349
- PMCID: PMC90362
- DOI: 10.1128/AAC.45.3.715-722.2001
Structural and functional analysis of horse cathelicidin peptides
Abstract
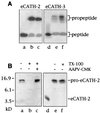
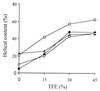
Cathelicidin-derived antimicrobial peptides are a component of the peptide-based host defense of neutrophils and epithelia, with a widespread distribution in mammals. We recently reported the cDNA sequences of three putative horse myeloid cathelicidins, named eCATH-1, -2, and -3. A Western analysis was performed to investigate their presence in neutrophils and processing to mature peptides. eCATH-2 and eCATH-3, but not eCATH-1, were found to be present in uncleaved forms in horse neutrophils. The corresponding mature peptides were detected in inflammatory sites, suggesting that processing of the propeptides takes place upon neutrophil activation. A functional characterization was then performed with synthetic eCATH peptides. Circular dichroism measurements indicated an amphipathic alpha-helical conformation of these peptides in an anisotropic environment, and in vitro assays revealed a potent activity and a broad spectrum of antimicrobial activity for eCATH-1 and a somewhat more restricted spectrum of activity for eCATH-2. Conversely, a strong dependence on salt concentration was observed when the activity of eCATH-3 was tested. This peptide efficiently killed bacteria and some fungal species, i.e., Cryptococcus neoformans and Rhodotorula rubra, in low-ionic-strength media, but the activity was inhibited in the presence of physiological salt medium. This behavior could be modified by modulating the amphipathicity of the molecule. In fact, the synthetic analogue LLK-eCATH-3, with a slightly modified sequence that increases the hydrophobic moment of the peptide, displayed a potent activity in physiological salt medium against the strains resistant to eCATH-3 under these conditions.
Figures



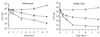
References
-
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson G H. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

