G(q/11) and G(i/o) activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype
- PMID: 11181437
- PMCID: PMC1572629
- DOI: 10.1038/sj.bjp.0703892
G(q/11) and G(i/o) activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype
Abstract
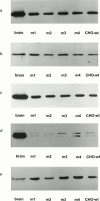
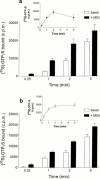
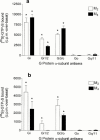
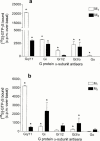
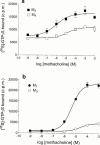
1. Profiles of G protein activation have been assessed using a [35S]-GTPgammaS binding/immunoprecipitation strategy in Chinese hamster ovary cells expressing either M1, M2, M3 or M4 muscarinic acetylcholine (mACh) receptor subtypes, where expression levels of M1 and M3, or M2 and M4 receptors were approximately equal. 2. Maximal [35S]-GTPgammaS binding to G(q/11)alpha stimulated by M1/M3 receptors, or G(i1-3)alpha stimulated by M2/M4 receptors occurred within approximately 2 min of agonist addition. The increases in G(q/11)alpha-[35S]-GTPgammaS binding after M1 and M3 receptor stimulation differed substantially, with M1 receptors causing a 2-3 fold greater increase in [35S]-GTPgammaS binding and requiring 5 fold lower concentrations of methacholine to stimulate a half-maximal response. 3. Comparison of M2 and M4 receptor-mediated G(i1-3)alpha-[35S]-GTPgammaS binding also revealed differences, with M2 receptors causing a greater increase in G(i1-3)alpha activation and requiring 10 fold lower concentrations of methacholine to stimulate a half-maximal response. 4. Comparison of methacholine- and pilocarpine-mediated effects revealed that the latter partial agonist is more effective in activating G(i3)alpha compared to G(i1/2)alpha for both M2 and M4 receptors. More marked agonist/partial agonist differences were observed with respect to M1/M3-mediated stimulations of G(q/11)alpha- and G(i1-3)alpha-[35S]-GTPgammaS binding. Whereas coupling to these Galpha subclasses decreased proportionately for M1 receptor stimulation by these agonists, pilocarpine possesses a greater intrinsic activity at M3 receptors for G(i)alpha versus G(q/11)alpha activation. 5. These data demonstrate that mACh receptor subtype and the nature of the agonist used govern the repertoire of G proteins activated. They also provide insights into how the diversity of coupling can be pharmacologically exploited, and provide a basis for a better understanding of how multiple receptor subtypes can be differentially regulated.
Figures

References
-
- AL-AOUKATY A., ROLSTAD B., MAGHAZACHI A.A. Functional coupling of NKR-P1 receptors to various heterotrimeric G proteins in rat interleukin-2-activated natural killer cells. J. Biol. Chem. 1997;272:31604–31608. - PubMed
-
- ARTHUR J.M., COLLINSWORTH G.P., GETTYS T.W., RAYMOND J.R. Agonist-induced translocation of Gq/11α immunoreactivity directly from plasma membrane in MDCK cells. Am. J. Physiol. 1999;276:F528–F534. - PubMed
-
- BARR A.J., BRASS L.F., MANNING D.R. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells: a direct evaluation of selectivity in receptor-G protein coupling. J. Biol. Chem. 1997;272:2223–2229. - PubMed
-
- BERG K.A., MAAYANI S., GOLDFARB J., SCARAMELLINI C., LEFF P., CLARKE W.P. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. - PubMed
-
- BERSTEIN G., BLANK J.L., SMRCKA A.V., HIGASHIJIMA T., STERNWEIS P.C., EXTON J.H., ROSS E.M. Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-β1. J. Biol. Chem. 1992;267:8081–8088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

