Rheumatoid arthritis synovial T cells regulate transcription of several genes associated with antigen-induced anergy
- PMID: 11181651
- PMCID: PMC199240
- DOI: 10.1172/JCI8027
Rheumatoid arthritis synovial T cells regulate transcription of several genes associated with antigen-induced anergy
Abstract
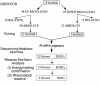
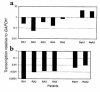
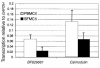
Rheumatoid arthritis (RA) is a chronic, inflammatory synovitis whose pathogenesis may involve autoimmune mechanisms. Anergy is a state of T-cell nonresponsiveness characterized by downregulated IL-2 production. Paradoxically, RA T cells are hyporesponsive and proliferate poorly to antigens and mitogens, thus sharing some characteristics with anergic T cells. We analyzed the molecular basis of anergy in cloned human CD4+ T cells using differential display RT-PCR and subsequently examined the levels of differentially expressed transcripts in RA and, as control, reactive arthritis (ReA) synovium. Several transcriptional events were common to anergic T cells and RA synovium. These included downregulation of CALMODULIN:, which is critical to T-cell activation, and of cellular apoptosis susceptibility protein, which may mediate resistance to apoptosis in RA. Transcription of CALMODULIN: in RA synovium was less than 1% of that in ReA and was lower in RA synovial fluid mononuclear cells than in paired PBMCs. Following anti-TNF-alpha therapy in vivo, RA PBMC CALMODULIN: transcripts increased five- to tenfold. Pharmacological calmodulin blockade in vitro impaired antigen-specific proliferation. These data provide a link between reduced CALMODULIN: transcription and impaired T-cell responsiveness in RA. The identification of transcriptional changes common to anergic and RA synovial T cells should help interpret some of the characteristic RA cellular defects.
Figures



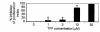
References
-
- Van Boxel JA, Paget SA. Predominantly T cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975; 293:517–520. - PubMed
-
- Fox DA. The role of T-cells in the immunopathogenesis of rheumatoid arthritis. Arthritis Rheum. 1997; 40:598–609. - PubMed
-
- Cush JJ, Lipsky PE. Phenotypic analysis of synovial and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988; 31:1230–1238. - PubMed
-
- Silverman HA, Johnson JS, Vaughan JH, McGlamory JC. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976; 19:509–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

