IkappaB kinase alpha is essential for mature B cell development and function
- PMID: 11181694
- PMCID: PMC2195900
- DOI: 10.1084/jem.193.4.417
IkappaB kinase alpha is essential for mature B cell development and function
Abstract
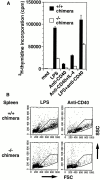
IkappaB kinase (IKK) alpha and beta phosphorylate IkappaB proteins and activate the transcription factor, nuclear factor (NF)-kappaB. Although both are highly homologous kinases, gene targeting experiments revealed their differential roles in vivo. IKKalpha is involved in skin and limb morphogenesis, whereas IKKbeta is essential for cytokine signaling. To elucidate in vivo roles of IKKalpha in hematopoietic cells, we have generated bone marrow chimeras by transferring control and IKKalpha-deficient fetal liver cells. The mature B cell population was decreased in IKKalpha(-/-) chimeras. IKKalpha(-/-) chimeras also exhibited a decrease of serum immunoglobulin basal level and impaired antigen-specific immune responses. Histologically, they also manifested marked disruption of germinal center formation and splenic microarchitectures that depend on mature B cells. IKKalpha(-/-) B cells not only showed impairment of survival and mitogenic responses in vitro, accompanied by decreased, although inducible, NF-kappaB activity, but also increased turnover rate in vivo. In addition, transgene expression of bcl-2 could only partially rescue impaired B cell development in IKKalpha(-/-) chimeras. Taken together, these results demonstrate that IKKalpha is critically involved in the prevention of cell death and functional development of mature B cells.
Figures





References
-
- Baeuerle P.A., Baltimore D. NF-κBten years after. Cell. 1996;87:13–20. - PubMed
-
- Barnes P.J., Karin M. Nuclear factor-κBa pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. - PubMed
-
- Regnier C.H., Song H.Y., Gao X., Goeddel D.V., Cao Z., Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. - PubMed
-
- Zandi E., Rothwarf D.M., Delhase M., Hayakawa M., Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. - PubMed
-
- Woronicz J.D., Gao X., Cao Z., Rothe M., Goeddel D.V. IκB kinase-βNF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

