Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice
- PMID: 11181698
- PMCID: PMC2195902
- DOI: 10.1084/jem.193.4.459
Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice
Abstract
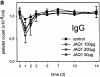
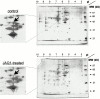
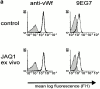
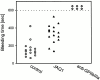
Coronary artery thrombosis is often initiated by abrupt disruption of the atherosclerotic plaque and activation of platelets on the subendothelial layers in the disrupted plaque. The extracellular matrix protein collagen is the most thrombogenic constituent of the subendothelial layer; therefore, a selective inhibition of the collagen activation pathway in platelets may provide strong antithrombotic protection while preserving other platelet functions. Here we demonstrate that treatment of mice with a monoclonal antibody against the activating platelet collagen receptor glycoprotein VI (GPVI; JAQ1) results in specific depletion of the receptor from circulating platelets and abolished responses of these cells to collagen and collagen-related peptides (CRPs). JAQ1-treated mice were completely protected for at least 2 wk against lethal thromboembolism induced by infusion of a mixture of collagen (0.8 mg/kg) and epinephrine (60 microg/ml). The tail bleeding times in JAQ1-treated mice were only moderately increased compared with control mice probably because the treatment did not affect platelet activation by other agonists such as adenosine diphosphate or phorbol myristate acetate. These results suggest that GPVI might become a target for long-term prophylaxis of ischemic cardiovascular diseases and provide the first evidence that it is possible to specifically deplete an activating glycoprotein receptor from circulating platelets in vivo.
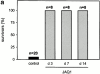
Figures














References
-
- Weiss H.J. Platelet physiology and abnormalities of platelet function. N. Engl. J. Med. 1975;293:531–541. - PubMed
-
- Weiss H.J. Platelet physiology and abnormalities of platelet function. N. Engl. J. Med. 1975;293:580–588. - PubMed
-
- Fuster V., Badimon L., Badimon J.J., Chesebro J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes. N. Engl. J. Med. 1992;326:242–250. - PubMed
-
- Fuster V., Badimon L., Badimon J.J., Chesebro J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes. N. Engl. J. Med. 1992;326:310–318. - PubMed
-
- Conti C.R., Mehta J.L. Acute myocardial ischemiarole of atherosclerosis, thrombosis, platelet activation, coronary vasospasm, and altered arachidonic acid metabolism. Circulation. 1987;75:V84–V95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

