Essential role of Glu-C66 for menaquinol oxidation indicates transmembrane electrochemical potential generation by Wolinella succinogenes fumarate reductase
- PMID: 11186225
- PMCID: PMC27176
- DOI: 10.1073/pnas.220425797
Essential role of Glu-C66 for menaquinol oxidation indicates transmembrane electrochemical potential generation by Wolinella succinogenes fumarate reductase
Abstract
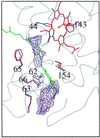
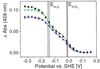
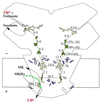
Quinol:fumarate reductase (QFR) is a membrane protein complex that couples the reduction of fumarate to succinate to the oxidation of quinol to quinone, in a reaction opposite to that catalyzed by the related enzyme succinate:quinone reductase (succinate dehydrogenase). In the previously determined structure of QFR from Wolinella succinogenes, the site of fumarate reduction in the flavoprotein subunit A of the enzyme was identified, but the site of menaquinol oxidation was not. In the crystal structure, the acidic residue Glu-66 of the membrane spanning, diheme-containing subunit C lines a cavity that could be occupied by the substrate menaquinol. Here we describe that, after replacement of Glu-C66 with Gln by site-directed mutagenesis, the resulting mutant is unable to grow on fumarate and the purified enzyme lacks quinol oxidation activity. X-ray crystal structure analysis of the Glu-C66-->Gln variant enzyme at 3.1-A resolution rules out any major structural changes compared with the wild-type enzyme. The oxidation-reduction potentials of the heme groups are not significantly affected. We conclude that Glu-C66 is an essential constituent of the menaquinol oxidation site. Because Glu-C66 is oriented toward a cavity leading to the periplasm, the release of two protons on menaquinol oxidation is expected to occur to the periplasm, whereas the uptake of two protons on fumarate reduction occurs from the cytoplasm. Thus our results indicate that the reaction catalyzed by W. succinogenes QFR generates a transmembrane electrochemical potential.
Figures

References
-
- Saraste M. Science. 1999;283:1488–1493. - PubMed
-
- Kröger A. Biochim Biophys Acta. 1978;505:129–145. - PubMed
-
- Kröger A, Geisler V, Lemma E, Theis F, Lenger R. Arch Microbiol. 1992;158:311–314.
-
- Hägerhäll C. Biochim Biophys Acta. 1997;1320:107–141. - PubMed
-
- Lancaster C R D, Kröger A, Auer M, Michel H. Nature (London) 1999;402:377–385. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

