HSP70 peptidembearing and peptide-negative preparations act as chaperokines
- PMID: 11189447
- PMCID: PMC312872
- DOI: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2
HSP70 peptidembearing and peptide-negative preparations act as chaperokines
Abstract
We recently elucidated a novel function for the 70-kDa heat shock protein (HSP70) as a chaperone and a cytokine, a chaperokine in human monocytes. Here we show that peptide-bearing and peptide-negative HSP70 preparations isolated from EMT6 mammary adenocarcinoma cells (EMT6-HSP70) act as chaperokines when admixed with murine splenocytes. EMT6-HSP70 bound with high affinity to the surface of splenocytes recovered from naive BALB/c mice. The [Ca2+]i inhibitor BAPTA dose dependently inhibited HSP70- but not LPS-induced NF-kappaB activity and subsequent augmentation of proinflammatory cytokine TNF-alpha, IL-1beta, and IL-6 production. Taken together, these results suggest that presence of peptide in the HSP70 preparation is not required for spontaneous activation of cells of the innate immune system.
Figures


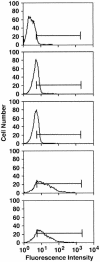
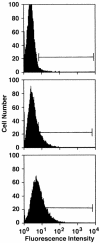

References
-
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, de laSalle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
-
- Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
