Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors
- PMID: 11191112
- PMCID: PMC1507984
- DOI: 10.1038/sj.neo.7900108
Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors
Abstract
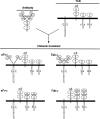
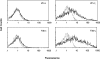
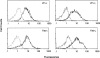
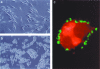
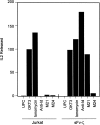
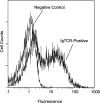
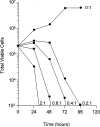
Immunoglobulin T-cell receptors (IgTCRs) combine the specificity of antibodies with the potency of cellular killing by grafting antibody recognition domains onto TCR signaling chains. IgTCR-modified T cells are thus redirected to kill tumor cells based on their expression of intact antigen on cell surfaces, bypassing the normal mechanism of activation through TCR-peptide-major histocompatibility complex (MHC) recognition. Melanoma is one of the most immunoresponsive of human cancers and has served as a prototype for the development of a number of immunotherapies. The target antigen for this study is the ganglioside GD3, which is highly expressed on metastatic melanoma with only minor immunologic cross-reaction with normal tissues. To determine an optimal configuration for therapy, four combinations of IgTCRs were prepared and studied: sFv-epsilon, sFv-zeta, Fab-epsilon, Fab-zeta. These were expressed on the surface of human T cells by retroviral transduction. IgTCR successfully redirected T-cell effectors in an MHC-unrestricted manner, in this case against a non-T-dependent antigen, with specific binding, activation, and cytotoxicity against GD3+ melanoma cells. Soluble GD3 in concentrations up to 100 microg/ml did not interfere with recognition and binding of membrane-bound antigen. Based on the outcomes of these structural and functional tests, the sFv-zeta construct was selected for clinical development. These results demonstrate key features that emphasize the potential of anti-GD3 IgTCR-modified autologous T cells for melanoma therapies.
Figures

Similar articles
-
Targeting of tumor cells by lymphocytes engineered to express chimeric receptor genes.Cancer Immunol Immunother. 2004 Oct;53(10):893-903. doi: 10.1007/s00262-004-0523-y. Epub 2004 May 26. Cancer Immunol Immunother. 2004. PMID: 15168086 Free PMC article. Review.
-
Bypassing immunization: optimized design of "designer T cells" against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA.Clin Cancer Res. 1999 Dec;5(12):3928-41. Clin Cancer Res. 1999. PMID: 10632322
-
Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors.Blood. 2002 Nov 1;100(9):3155-63. doi: 10.1182/blood-2002-04-1041. Blood. 2002. PMID: 12384413
-
Specific lysis of melanoma cells by receptor grafted T cells is enhanced by anti-idiotypic monoclonal antibodies directed to the scFv domain of the receptor.J Invest Dermatol. 1999 May;112(5):744-50. doi: 10.1046/j.1523-1747.1999.00586.x. J Invest Dermatol. 1999. PMID: 10233766
-
Adoptive immuno-gene therapy of cancer with single chain antibody [scFv(Ig)] gene modified T lymphocytes.J Biol Regul Homeost Agents. 2004 Apr-Jun;18(2):134-40. J Biol Regul Homeost Agents. 2004. PMID: 15471217 Review.
Cited by
-
Proteomic footprinting of drug-treated cancer cells as a measure of cellular vaccine efficacy for the prevention of cancer recurrence.Mol Cell Proteomics. 2012 Feb;11(2):M111.014480. doi: 10.1074/mcp.M111.014480. Epub 2011 Nov 9. Mol Cell Proteomics. 2012. PMID: 22074704 Free PMC article.
-
Allogeneic Antigen Composition for Preparing Universal Cancer Vaccines.J Immunol Res. 2016;2016:5031529. doi: 10.1155/2016/5031529. Epub 2016 Oct 3. J Immunol Res. 2016. PMID: 27781211 Free PMC article.
-
Targeting of tumor cells by lymphocytes engineered to express chimeric receptor genes.Cancer Immunol Immunother. 2004 Oct;53(10):893-903. doi: 10.1007/s00262-004-0523-y. Epub 2004 May 26. Cancer Immunol Immunother. 2004. PMID: 15168086 Free PMC article. Review.
-
Cell Proteomic Footprinting: Advances in the Quality of Cellular and Cell-Derived Cancer Vaccines.Pharmaceutics. 2023 Feb 16;15(2):661. doi: 10.3390/pharmaceutics15020661. Pharmaceutics. 2023. PMID: 36839983 Free PMC article. Review.
-
Benign tumors in TSC are amenable to treatment by GD3 CAR T cells in mice.JCI Insight. 2021 Nov 22;6(22):e152014. doi: 10.1172/jci.insight.152014. JCI Insight. 2021. PMID: 34806651 Free PMC article.
References
-
- Chapman PB, Lonberg M, Houghton AN. Light chain variants of an IgG3 anti-GD3 monoclonal antibody and the relationship among avidity, effector functions, tumor targeting, and antitumor activity. Cancer Res. 1990;50:1503–1509. - PubMed
-
- Portoukalian J, Carrel S, Dore JF, Rumke P. Humoral immune response in disease-free advanced melanoma patients after vaccination with melanoma-associated gangliosides. EORTC Cooperative Melanoma Group. Int J Cancer. 1991;49:893–899. - PubMed
-
- Cheresh DA. Human melanoma cell attachment involves an Arg-Gly-Asp-directed adhesion receptor and the disialoganglioside GD2. Prog Clin Biol Res. 1989;288:3–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
