Prediction of the interacting surfaces in a trimolecular complex formed between the major dust mite allergen Der p 1, a mouse monoclonal anti-Der p 1 antibody, and its anti-idiotype
- PMID: 11193052
- PMCID: PMC1186988
- DOI: 10.1136/mp.53.6.324
Prediction of the interacting surfaces in a trimolecular complex formed between the major dust mite allergen Der p 1, a mouse monoclonal anti-Der p 1 antibody, and its anti-idiotype
Abstract
Background: Two mouse monoclonal antibodies (mAbs) have been described recently; namely, mAb 2C7 (IgG2b kappa), which is directed against the major house dust mite allergen Der p 1, and mAb 2G10 (IgG1 kappa), which is an anti-idiotypic antibody raised against mAb 2C7. The anti-idiotype mAb 2G10 does not block the binding of mAb 2C7 to Der p 1, which means that mAb 2C7 can simultaneously bind to Der p 1 and to mAb 2G10, thereby generating a trimolecular complex consisting of antigen-idiotype-anti-idiotype.
Aims: To sequence and model the V region of the anti-idiotypic antibody mAb 2G10 to enable the prediction of the interacting surfaces in the trimolecular complex consisting of Der p 1-mAb 2C7-mAb 2G10.
Methods: DNA sequencing of mAb 2G10 was carried out and the Swiss Model and Swiss PDB-Viewer programs were used to build a three dimensional model of the trimolecular complex.
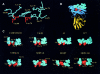
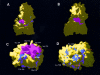
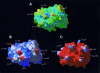
Results: Complementarity of shape and charge was revealed when comparing the protrusion of the previously determined Der p 1 epitope (Leu147-Gln160) with the cavity formed by the complementarity determining regions (CDRs) of mAb 2C7. Such complementarity was also observed between the mAb 2C7 epitope predicted to be recognised by mAb 2G10 (residues Lys19 from framework region 1 (FRW1) and Ser74-Gln81 from FRW3) and residues from the CDRs of mAb 2G10 (a negatively charged patch flanked by the residues Asp55H/Glu58H and Glu27L/Glu27cL). As expected, the location of the mAb 2C7 epitope recognised by mAb 2G10 does not appear to interfere with the binding of Der p 1 to mAb 2C7.
Conclusion: Although the results obtained represent only an approximation, they nevertheless provide a rare insight into how an antigen (Der p 1) might bind to its antibody (mAb 2C7) while in complex with an anti-idiotype (mAb 2G10).
Figures

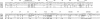
Similar articles
-
The production and characterisation of a chimaeric human IgE antibody, recognising the major mite allergen Der p 1, and its chimaeric human IgG1 anti-idiotype.Mol Pathol. 2002 Oct;55(5):315-24. doi: 10.1136/mp.55.5.315. Mol Pathol. 2002. PMID: 12354937 Free PMC article.
-
Characterisation of a mouse monoclonal anti-idiotype reactive with a V region sequence commonly used by human immunoglobulins.Mol Pathol. 2000 Apr;53(2):77-82. doi: 10.1136/mp.53.2.77. Mol Pathol. 2000. PMID: 10889906 Free PMC article.
-
The use of phage-peptide libraries to define the epitope specificity of a mouse monoclonal anti-Der p 1 antibody representative of a major component of the human immunoglobulin E anti-Der p 1 response.Clin Exp Allergy. 1999 Nov;29(11):1563-71. doi: 10.1046/j.1365-2222.1999.00686.x. Clin Exp Allergy. 1999. PMID: 10520087
-
Primary sequence and molecular model of the variable region of a mouse monoclonal anti-Der p 1 antibody showing a similar epitope specificity as human IgE.Clin Exp Allergy. 1998 Nov;28(11):1427-34. doi: 10.1046/j.1365-2222.1998.00378.x. Clin Exp Allergy. 1998. PMID: 9824417
-
Augmentation of tumor antigen expression by recombinant human interferons: enhanced targeting of monoclonal antibodies to carcinomas.Cancer Treat Res. 1990;51:413-32. doi: 10.1007/978-1-4613-1497-4_21. Cancer Treat Res. 1990. PMID: 1977458 Review.
Cited by
-
The production and characterisation of a chimaeric human IgE antibody, recognising the major mite allergen Der p 1, and its chimaeric human IgG1 anti-idiotype.Mol Pathol. 2002 Oct;55(5):315-24. doi: 10.1136/mp.55.5.315. Mol Pathol. 2002. PMID: 12354937 Free PMC article.
-
Homology modelling: a review about the method on hand of the diabetic antigen GAD 65 structure prediction.Wien Med Wochenschr. 2009;159(5-6):112-25. doi: 10.1007/s10354-009-0662-z. Wien Med Wochenschr. 2009. PMID: 19343288 Review.
References
-
- McElveen JE, Clark MR, Smith SJ, et al. Primary sequence and molecular model of the variable region of a mouse monoclonal anti-Der p 1 antibody showing a similar epitope specificity as human IgE. Clin Exp Allergy 1998;28:1427–34. - PubMed
-
- Furmonaviciene R, Tighe PJ, Clark MR, et al. The use of phage-peptide libraries to define the epitope specificity of a mouse monoclonal anti-Der p1 antibody representative of a major component of the human immunoglobulin E anti-Der p 1 response. Clin Exp Allergy 1999;29:1563–71. - PubMed
-
- Riechmann L, Clark MR, Waldmann H, et al. Reshaping human antibodies for therapy. Nature 1988;332: 323–7. - PubMed
-
- Evans SV, Rose DR, To R, et al. Exploring the mimicry of polysaccharide antigens by anti-idiotypic antibodies. The crystallization, molecular replacement, and refinement to 2.8 Å resolution of an idiotope–anti-idiotope Fab complex and of the unliganded anti-idiotope Fab. J Mol Biol 1994;241:691–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
