Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA
- PMID: 11208794
- PMCID: PMC94963
- DOI: 10.1128/JB.183.3.951-958.2001
Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA
Abstract
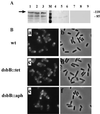
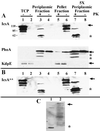
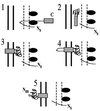
The Shigella outer membrane protein IcsA belongs to the family of type V secreted (autotransported) virulence factors. Members of this family mediate their own translocation across the bacterial outer membrane: the carboxy-terminal beta domain forms a beta barrel channel in the outer membrane through which the amino-terminal alpha domain passes. IcsA, which is localized at one pole of the bacterium, mediates actin assembly by Shigella, which is essential for bacterial intracellular movement and intercellular dissemination. Here, we characterize the transit of IcsA across the periplasm during its secretion. We show that an insertion in the dsbB gene, whose gene product mediates disulfide bond formation of many periplasmic intermediates, does not affect the surface expression or unipolar targeting of IcsA. However, IcsA forms one disulfide bond in the periplasm in a DsbA/DsbB-dependent fashion. Furthermore, cellular fractionation studies reveal that IcsA has a transient soluble periplasmic intermediate. Our data also suggest that IcsA is folded in a proteinase K-resistant state in the periplasm. From these data, we propose a novel model for the secretion of IcsA that may be applicable to other autotransported proteins.
Figures




References
-
- Allaoui A, Mounier J, Prevost M-C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. - PubMed
-
- Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. - PubMed
-
- Bartolome B, Jubete Y, Martinez E, de-la-Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

