Cytoplasmic filament-deficient mutant of Treponema denticola has pleiotropic defects
- PMID: 11208807
- PMCID: PMC94976
- DOI: 10.1128/JB.183.3.1078-1084.2001
Cytoplasmic filament-deficient mutant of Treponema denticola has pleiotropic defects
Abstract
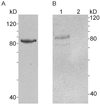
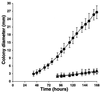
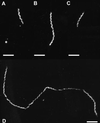
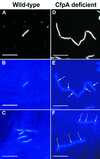
In Treponema denticola, a ribbon-like structure of cytoplasmic filaments spans the cytoplasm at all stages of the cell division process. Insertional inactivation was used as a first step to determine the function of the cytoplasmic filaments. A suicide plasmid was constructed that contained part of cfpA and a nonpolar erythromycin resistance cassette (ermF and ermAM) inserted near the beginning of the gene. The plasmid was electroporated into T. denticola, and double-crossover recombinants which had the chromosomal copy of cfpA insertionally inactivated were selected. Immunoblotting and electron microscopy confirmed the lack of cytoplasmic filaments. The mutant was further analyzed by dark-field microscopy to determine cell morphology and by the binding of two fluorescent dyes to DNA to assess the distribution of cellular nucleic acids. The cytoplasmic filament protein-deficient mutant exhibited pleiotropic defects, including highly condensed chromosomal DNA, compared to the homogeneous distribution of the DNA throughout the cytoplasm in a wild-type cell. Moreover, chains of cells are formed by the cytoplasmic filament-deficient mutant, and those cells show reduced spreading in agarose, which may be due to the abnormal cell length. The chains of cells and the highly condensed chromosomal DNA suggest that the cytoplasmic filaments may be involved in chromosome structure, segregation, or the cell division process in Treponema.
Figures



Similar articles
-
Genetic and structural analyses of cytoplasmic filaments of wild-type Treponema phagedenis and a flagellar filament-deficient mutant.J Bacteriol. 1999 Nov;181(21):6739-46. doi: 10.1128/JB.181.21.6739-6746.1999. J Bacteriol. 1999. PMID: 10542176 Free PMC article.
-
Insertional inactivation of Treponema denticola tap1 results in a nonmotile mutant with elongated flagellar hooks.J Bacteriol. 1999 Jun;181(12):3743-50. doi: 10.1128/JB.181.12.3743-3750.1999. J Bacteriol. 1999. PMID: 10368149 Free PMC article.
-
Insertional inactivation of the prtP gene of Treponema denticola confirms dentilisin's disruption of epithelial junctions.J Mol Microbiol Biotechnol. 2000 Oct;2(4):581-6. J Mol Microbiol Biotechnol. 2000. PMID: 11075935
-
Cytoskeletal cytoplasmic filament ribbon of Treponema: a member of an intermediate-like filament protein family.J Mol Microbiol Biotechnol. 2006;11(3-5):159-66. doi: 10.1159/000094052. J Mol Microbiol Biotechnol. 2006. PMID: 16983193 Review.
-
Bacterial chromosome dynamics.Science. 2003 Aug 8;301(5634):780-5. doi: 10.1126/science.1084780. Science. 2003. PMID: 12907786 Review.
Cited by
-
Major membrane protein TDE2508 regulates adhesive potency in Treponema denticola.PLoS One. 2014 Feb 21;9(2):e89051. doi: 10.1371/journal.pone.0089051. eCollection 2014. PLoS One. 2014. PMID: 24586498 Free PMC article.
-
Pathogenicity of Treponema denticola Wild-Type and Mutant Strain Tested by an Active Mode of Periodontal Infection Using Microinjection.Int J Dent. 2012;2012:549169. doi: 10.1155/2012/549169. Epub 2012 Jul 5. Int J Dent. 2012. PMID: 22829826 Free PMC article.
-
Deciphering morphological determinants of the helix-shaped Leptospira.J Bacteriol. 2011 Nov;193(22):6266-75. doi: 10.1128/JB.05695-11. Epub 2011 Sep 16. J Bacteriol. 2011. PMID: 21926230 Free PMC article.
-
Killing of Treponema denticola by mouse peritoneal macrophages.J Dent Res. 2010 May;89(5):521-6. doi: 10.1177/0022034510363105. Epub 2010 Mar 3. J Dent Res. 2010. PMID: 20200417 Free PMC article.
-
Electron cryotomography: a new view into microbial ultrastructure.Curr Opin Microbiol. 2009 Jun;12(3):333-40. doi: 10.1016/j.mib.2009.03.007. Epub 2009 May 6. Curr Opin Microbiol. 2009. PMID: 19427259 Free PMC article. Review.
References
-
- Akerlund T, Bernander R, Nordstrom K. Cell division in Escherichia coli minB mutants. Mol Microbiol. 1992;6:2073–2083. - PubMed
-
- Berg H C. How spirochetes may swim. J Theor Biol. 1976;56:269–273. - PubMed
-
- Bermudes D, Chase D, Margulis L. Morphology as a basis for taxonomy of large spirochetes symbiotic in wood-eating cockroaches and termites: Pillotina gen. nov., nom. rev.; Pillotina calotermitidis sp. nov., nom. rev.; Diplocalyx gen. nov., nom. rev.; Diplocalyx calotermitidis sp. nov., nom. rev.; Hollandina gen. nov., nom. rev.; Hollandina pterotermitidis sp. nov., nom. rev.; and Clevelandina reticulitermitidis gen. nov., sp. nov. Int J Syst Bacteriol. 1988;38:291–302. - PubMed
-
- Bi E F, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

