Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen
- PMID: 11208980
- PMCID: PMC2278413
- DOI: 10.1111/j.1469-7793.2001.0331l.x
Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen
Abstract
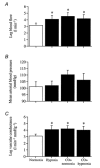
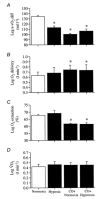
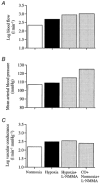
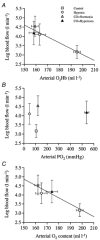
We hypothesised that reducing arterial oxyhaemoglobin (O2Hba) with carbon monoxide (CO) in both normoxia and hyperoxia, or acute hypoxia would cause similar compensatory increases in human skeletal muscle blood flow and vascular conductance during submaximal exercise, despite vast differences in arterial free oxygen partial pressure (Pa,O2). Seven healthy males completed four 5 min one-legged knee-extensor exercise bouts in the semi-supine position (30 +/- 3 W, mean +/- S.E.M.), separated by approximately 1 h of rest, under the following conditions: (a) normoxia (O2Hba = 195 ml l-1; Pa,O2 = 105 mmHg); (b) hypoxia (163 ml l-1; 47 mmHg); (c) CO + normoxia (18% COHba; 159 ml l-1; 119 mmHg); and (d) CO + hyperoxia (19% COHba; 158 ml l-1; 538 mmHg). CO + normoxia, CO + hyperoxia and systemic hypoxia resulted in a 29-44% higher leg blood flow and leg vascular conductance compared to normoxia (P < 0.05), without altering blood pH, blood acid-base balance or net leg lactate release. Leg blood flow and leg vascular conductance increased in association with reduced O2Hba (r2 = 0.92-0.95; P < 0.05), yet were unrelated to altered Pa,O2. This association was further substantiated in two subsequent studies with graded increases in COHba (n = 4) and NO synthase blockade (n = 2) in the presence of normal Pa,O2. The elevated leg blood flow with CO + normoxia and CO + hyperoxia allowed a approximately 17% greater O2 delivery (P < 0.05) to exercising muscles, compensating for the lower leg O2 extraction (61%) compared to normoxia and hypoxia (69%; P < 0.05), and thereby maintaining leg oxygen uptake constant. The compensatory increases in skeletal muscle blood flow and vascular conductance during exercise with both a CO load and systemic hypoxia are independent of pronounced alterations in Pa,O2 (47-538 mmHg), but are closely associated with reductions in O2Hba. These results suggest a pivotal role of O2 bound to haemoglobin in increasing skeletal muscle vasodilatation during exercise in humans.
Figures


References
-
- Asmussen E, Nielsen M. The cardiac output in rest and work at low and high oxygen pressures. Acta Physiologica Scandinavica. 1955;35:73–83. - PubMed
-
- Asmussen E, Vinther-Paulsen N. On the circulatory adaptations to arterial hypoxemia (CO-poisoning) Acta Physiologica Scandinavica. 1949;19:115–124.
-
- Baron JF, Vicaut E, Hou X, Duvelleroy M. Independent role of arterial O2 tension in local control of coronary blood flow. American Journal of Physiology. 1990;258:H1388–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

