Refined crystal structure of DsRed, a red fluorescent protein from coral, at 2.0-A resolution
- PMID: 11209050
- PMCID: PMC14609
- DOI: 10.1073/pnas.98.2.462
Refined crystal structure of DsRed, a red fluorescent protein from coral, at 2.0-A resolution
Abstract
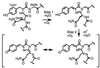
The crystal structure of DsRed, a red fluorescent protein from a corallimorpharian, has been determined at 2.0-A resolution by multiple-wavelength anomalous dispersion and crystallographic refinement. Crystals of the selenomethionine-substituted protein have space group P2(1) and contain a tetramer with 222 noncrystallographic symmetry in the asymmetric unit. The refined model has satisfactory stereochemistry and a final crystallographic R factor of 0.162. The protein, which forms an obligatory tetramer in solution and in the crystal, is a squat rectangular prism comprising four protomers whose fold is extremely similar to that of the Aequorea victoria green fluorescent protein despite low ( approximately 23%) amino acid sequence homology. The monomer consists of an 11-stranded beta barrel with a coaxial helix. The chromophores, formed from the primary sequence -Gln-Tyr-Gly- (residues 66-68), are arranged in a approximately 27 x 34-A rectangular array in two approximately antiparallel pairs. The geometry at the alpha carbon of Gln-66 (refined without stereochemical restraints) is consistent with an sp(2) hybridized center, in accord with the proposal that red fluorescence is because of an additional oxidation step that forms an acylimine extension to the chromophore [Gross, L. A., Baird, G. S., Hoffman, R. C., Baldridge, K. K. & Tsien, R. Y. (2000) Proc. Natl. Acad. Sci. USA 87, 11990-11995]. The carbonyl oxygen of Phe-65 is almost 90 degrees out of the plane of the chromophore, consistent with theoretical calculations suggesting that this is the minimum energy conformation of this moiety despite the conjugation of this group with the rest of the chromophore.
Figures




References
-
- Tsien R Y. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Remington S J. In: Bioluminescence and Chemiluminescence. Baldwin T O, Sigler M M, editors. Vol. 305. San Diego: Academic; 2000. pp. 195–211.
-
- Lukyanov K A, Fradkov A F, Gurskaya N G, Matz M V, Markelov M L, Zaraisky A G, Zhao X, Fang Y, Tan W, Lukyanov S A. J Biol Chem. 2000;275:25879–25882. - PubMed
-
- Matz M V, Arkady F F, Labas Y A, Savitsky A P, Zaraisky A G, Markelov M L, Lukyanov S A. Nat Biotechnol. 1999;17:969–973. - PubMed
-
- Dove S, Takabayashi M, Hoegh-Guldberg O. Biol Bull. 1995;189:288–297. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

